Generality
What is it, but above all, why is lactic acid produced by cells?
Lactic acid (C 3 H 6 O 3 ) is a weak acid that is produced by cells that extract energy through anaerobic glycolysis, therefore by breaking down glucose in the absence of oxygen. To be precise, anaerobic glycolysis is an essential process that precedes the Krebs cycle and therefore constitutes a fundamental step in cellular respiration; therefore, why sometimes anaerobic glycolysis reaches the production of lactic acid instead of continuing cellular respiration with pyruvate? Simple, WHEN the energy demand is urgent and / or the availability of oxygen is scarce there is an accumulation of NADH in the Krebs cycle (reduced NAD); consequently, anaerobic glycolysis adds another step: the transfer of a hydrogen cation from NADH to pyruvate which becomes lactic acid . The production of lactic acid is the fastest method to continue to derive energy if the aerobic metabolism does not have a sufficient availability of NAD transporters.
In which districts is the greatest amount of lactic acid released? And where does it end?
The quantity of lactic acid normally produced and tolerated by an adult organism is about 5-18mg / ml of blood (0.05-0.18mg / dl), but if the accumulation crosses this threshold it MAY be harmful to health. The excess lactic acid (contained in the districts that produce it) enters the capillaries and pours into the circulatory stream quickly reaching the liver, which uses it to obtain again some pyruvate and then glucose (neoglucogenesis or Cori cycle).
The tissue that produces the most lactic acid is the striated muscle; this consequently happens to the great energy demand necessary for athletic and sporting efforts, both prolonged and over anaerobic threshold, both short and super-intense (> 12-17 seconds, once creatine phosphate - CP is terminated).
In addition to intense physical activity, there are other causes of production and accumulation of lactic acid; some of them are:
- Cancer
- AIDS
- Taking biguanide drugs (ANTI-hyperglycemic agents)
- Idiopathic (whose causes are unknown)
- Mitochondrial diseases (genetics)
- Excessive alcohol ingestion (in which the buffer mechanisms are not able to meet the needs of acidosis)
- Anemia
- Liver cirrhosis (lack of liver function to carry out neoglucogenesis)
- Diabetes
- Renal conic insufficiency (reduced secretion of bicarbonates)
- Respiratory crisis.
It is quite interesting to try to understand how an accumulation of lactic acid can occur due to a respiratory crisis. This serious symptom can be associated with: bronchoconstriction, pulmonary edema, physical airway obstruction, etc., resulting in a significant reduction in ventilation-perfusion, and therefore in peripheral oxygenation. In such circumstances, ALL oxygen-deficient districts try to "get by" restoring NAD by lactic dehydrogenase (lactic acid production from pyruvate); this explains why, in respiratory crises, there is a surge of blood lactic acid, sometimes followed by the hypoxic blackout, or worse, by a syncopal state.
Curiosity
We have already anticipated that lactic acid is secreted in greater quantities in the event of excessive energy demand for aerobic metabolism or lack of oxygen to the tissues; but adding both variables, what would happen? It is not difficult to understand that, among ordinary people, this is a more unique than rare event ... yet some are exposed to it voluntarily, repeatedly and with enthusiasm! This is the case with underwater freedivers.
Underwater apnea, in the disciplines of constant attitude and dynamic apnea, provides a relatively intense muscular effort (different between the two specialties) BUT ALWAYS in the absence of pulmonary ventilation; not by chance, in the training of these disciplines, the components of production and tolerance to lactate, and the specific property of muscular disposal, are qualities to say the least essential.
Lactate threshold
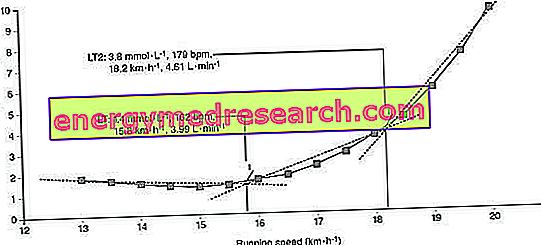
The evaluation of blood lactate is also used in sports performance tests. During an incremental exercise (which involves increases in intensity at predetermined time intervals, up to the maximum effort), the concentrations of lactic acid in the blood follow a trend similar to that shown in the figure. The first point of deflection (LT) represents the subject's aerobic threshold, or the point beyond which lactic acid begins to accumulate in the blood; the aerobic threshold is made to coincide with lactacidemia levels of 2-2.5 mM / l; in this phase the energetic mixture consumed by the athlete is predominantly lipidic (in trained athletes) and the intensity of the exercise can be maintained for a long time (several hours), since the increased acidity of the blood is compensated by an increase in the ventilatory activity. The second point of deflection represents instead the anaerobic threshold, which corresponds to an intensity of exercise to which the accumulation of lactic acid becomes particularly important, such as to impose a decrease in the intensity of exercise within a time interval (10-30 minutes, an interval that increases as the athlete's training increases). The anaerobic threshold is concido to lacticidemia levels equal to (3.9-4 mM / l) and foresees the prevalent consumption of glucose obtained from muscle glycogen.
Metabolic fate
How does the body react to excessive lactic acid production?
We have specified that lactic acid, despite being a toxic catabolite, can be reused by the body. This happens in different ways: inside the tissues themselves (in the muscle fibrocells, in particular the red ones for the conversion of lactate into pyruvate), in other tissues (such as the cardiac one, able to use DIRECTLY the lactate itself), or in the liver for neoglucogenesis.
Contrary to what many STILL believe (it is a banal commonplace), RARELY the lactic acid "stagnates" in the circulation or in the tissues for more than 2 hours after its release; in fact, in most cases, its concentrations (as high as they are) are significantly reduced within 60 'from the end of the exercises. Therefore, the pains suffered as a result of super intense exercises are due exclusively to micro-lesions of the muscle tissue and the inflammatory-like stimulus they exert on the nerve endings.
The ability to dispose of lactic acid is ALLENABLE and depends essentially on biochemical-enzymatic, muscular and hepatic factors.
To counteract the lowering of the pH there is also a system of contrast of cellular and sanguine acidity, or the buffer system; this is essentially based on the secretion of bicarbonate / carbonic acid which, thanks to its chemical peculiarities, buffers the hydrogen ions (H +) released by the dissociation of the circulating lactic acid with the production of carbon dioxide (CO 2, subsequently expelled by means of pulmonary ventilation) and water (H 2 O).



NB . Sodium bicarbonate (domestic product) is still considered a food supplement that counteracts blood acidosis; however, although it has shown some positive effects, it also has negative effects of significant importance; for further information, read the article: Lactic Acid Remedies - Supplements, Diet.