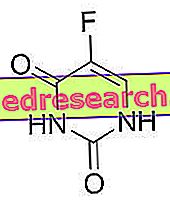
Flumazenil is an antagonist of the benzodiazepine binding site on the GABA-A receptor. From the chemical point of view, flumazenil is an imidazobenzodiazepine.

Flumazenil - Chemical Structure
Indications
For what it uses
Flumazenil is able to neutralize the central sedative effects induced by benzodiazepines and benzodiazepine-like substances (such as, for example, zolpidem). Therefore, flumazenil is used in the following conditions:
- Interruption of general anesthesia induced or maintained by benzodiazepines in both hospitalized and outpatient patients;
- Antidote in case of overdose - accidental or voluntary - of benzodiazepines or benzodiazepine-like drugs;
- Neutralization of paradox reactions due to benzodiazepines or benzodiazepine-like substances.
Warnings
Flumazenil should not be used for the treatment of benzodiazepine dependence and for the treatment of benzodiazepine withdrawal syndrome.
After administration of flumazenil, patients should be monitored for at least 24 hours.
Patients with chronic or transient anxiety require adjustment of the administered flumazenil dose.
The administration of flumazenil in patients who have taken benzodiazepines at high doses and for a long time can cause withdrawal symptoms.
Flumazenil should be used with caution in neutralizing sedation in children and in resuscitation of the newborn.
When used to stop anesthesia after surgery, flumazenil should only be administered when the muscle relaxant effect on peripheral muscle has disappeared.
Flumazenil elimination is slowed in patients with impaired liver function.
A lot of caution should be used when administering flumazenil in overdosed patients with benzodiazepines and antidepressants, since the administration of the drug could favor the appearance - or the deterioration - of convulsions and cardiac arrhythmias.
Administration of flumazenil in epileptic patients on benzodiazepine therapy for a long time is not recommended, as seizures and seizures may occur.
When flumazenil is given as an antidote to overdosage with benzodiazepines or benzodiazepine-like substances, patients must be carefully monitored for a certain period of time. This period varies depending on the type and duration of action of the hypnotic drug taken.
Flumazenil should be administered with caution in patients suffering from brain lesions treated with benzodiazepines, since - in this category of patients - it can induce seizures and alterations in cerebral blood flow. Furthermore, an increase in intracranial pressure may also occur.
Although flumazenil stops the sedative effect, it is advisable not to drive or use machines for at least 24 hours after taking the drug.
Interactions
No interactions were found between flumazenil and other drugs with depressive action on the central nervous system.
There are no known drug interactions between flumazenil and alcohol.
Side effects
Flumazenil is well tolerated in both adults and children, but it can induce various types of side effects, even if not all patients experience them.
The following are the main adverse effects that may occur following the administration of flumazenil.
Psychiatric disorders
With a rapid injection of flumazenil withdrawal symptoms can occur, such as:
- agitation;
- Anxiety;
- Confusion;
- Emotional ability;
- Sensory distortion.
Furthermore, panic attacks, abnormal crying and aggressive reactions can occur.
Nervous system disorders
Following administration of flumazenil, seizures or seizures may occur, especially in patients with prior epilepsy or severe hepatic impairment.
Seizures occur more easily in those patients who have been treated with benzodiazepines for long periods or in patients overdosed on multiple drugs.
Cardiac disorders
When flumazenil is given through a rapid injection, it could cause palpitations. Generally, this adverse effect does not require pharmacological treatment.
Vascular pathologies
After administration of flumazenil, patients may experience a transient increase in blood pressure upon awakening from anesthesia.
Gastrointestinal disorders
During post-operative use of flumazenil, nausea and vomiting may occur. This happens especially if opiate medicines have also been administered.
Other side effects
Other adverse effects that may occur following administration of flumazenil are:
- Hypersensitivity reactions - including anaphylaxis - in sensitive subjects;
- Redness of the skin;
- Chills, following a rapid injection.
Overdose
There is no antidote for flumazenil overdose.
In case of overdose, the vital signs of the patients must be monitored and the therapy is only supportive.
However, following intake of flumazenil amounts above the usual doses, no symptoms due to overdose have been reported.
Action mechanism
As mentioned above, flumazenil is an antagonist of the benzodiazepine binding site present on the GABA-A receptor. In particular, flumazenil competes with benzodiazepines and non-benzodiazepine agonists for binding to the aforementioned site.
Occupying the binding site on GABA-A, flumazenil is thus able to neutralize sedation, amnesia, psychomotor alteration and respiratory depression induced by this type of hypnotic drugs.
Mode of Use - Posology
Flumazenil is available for intravenous administration.
The drug should only be administered by an anesthesiologist or a specialist.
In patients with hepatic impairment an adjustment of the usual administered dose is necessary.
Interruption of anesthesia
For discontinuation of anesthesia the dose of flumazenil usually administered is 0.3-0.6 mg.
The maximum dose that can be administered is 1 mg of the drug.
In children over 1 year of age, the recommended flumazenil dose is 0.01 mg / kg body weight.
Antidote in case of overdosage with benzodiazepines or benzodiazepine-like drugs
In this case, the recommended flumazenil dose is 0.3 mg.
The maximum dose that can be administered is 2 mg of the drug.
Pregnancy and breastfeeding
There are insufficient data to establish the safety of the use of flumazenil in pregnancy. Therefore, flumazenil should be administered during pregnancy only if the expected benefits to the mother outweigh the potential risks to the fetus.
It is not known whether flumazenil is excreted in breast milk. Intravenous administration of flumazenil in breastfeeding mothers is not contraindicated, but it is recommended to avoid breastfeeding for the 24 hours following the administration of the drug.
Contraindications
The use of flumazenil is contraindicated in the following cases:
- Known hypersensitivity to flumazenil;
- In patients in whom benzodiazepines have been administered to control a severe epileptic seizure or to control intracranial pressure;
- In patients with mixed poisoning from benzodiazepines and tricyclic and tetracyclic antidepressants, such as imipramine, clomipramine, mirtazapine and mianserin.