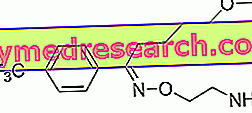
What is Libertek - roflumilast?
Libertek is a medicine that contains the active substance roflumilast. The medicine is available as yellow D-shaped tablets (500 micrograms).
This medicine is similar to Daxas, which is already authorized in the European Union (EU). The company that makes Daxas considered that the scientific data related to it could also be used for Libertek ("informed consent").
What is Libertek - roflumilast used for?
Libertek is used to treat chronic obstructive pulmonary disease (COPD) in adults with chronic bronchitis (chronic airway inflammation) and with frequent exacerbations of COPD. COPD is a chronic disease in which the airways and pulmonary alveoli are damaged or blocked, resulting in difficulty in inhaling and exhaling air from the lungs.
Libertek is not used alone but "added" to treatment with bronchodilators (medicines that widen the airways of the lungs).
The medicine can only be obtained with a prescription.
How is Libertek - roflumilast used?
The recommended dose of Libertek is one tablet once a day. The tablets should be taken with a little water every day at the same time. Patients may have to take Libertek for a few weeks before it starts to take effect.
How does Libertek - roflumilast work?
The active substance in Libertek, roflumilast, belongs to a group of medicines called "phosphodiesterase type 4 inhibitors (PDE4)". It blocks the action of the PDE4 enzyme that participates in the inflammatory process that leads to COPD. By blocking the action of PDE4, roflumilast reduces inflammation in the lungs helping to alleviate the patient's symptoms and prevent them from worsening.
How has Libertek - roflumilast been studied?
The effects of Libertek were first tested in experimental models before being studied in humans.
Libertek has been compared with placebo (a dummy treatment) in two main studies involving a total of over 3, 000 adults with severe COPD who had had at least one exacerbation of the disease in the past year. During the study, patients could continue to receive treatment with a bronchodilator. The main measure of effectiveness was the improvement in forced expiratory volume (FEV1) and the reduction in the number of moderate or severe COPD exacerbations during one year of treatment. The FEV1 is the maximum air that a person can breathe out in a second.
What benefit has Libertek - roflumilast shown during the studies?
Libertek was more effective than placebo in the treatment of COPD. At the beginning of the study, the two groups of patients had an FEV1 of about 1 liter (1, 000 ml). After one year, patients taking Libertek had a mean increase of 40 ml while those receiving placebo had a mean reduction of 9 ml. Furthermore, patients taking Libertek had an average of 1.1 moderate or severe exacerbations of the disease, compared to the 1.4 exacerbations of patients taking placebo.
What is the risk associated with Libertek - roflumilast?
The most common side effects of Libertek (seen in between 1 and 10 patients in 100) are weight and appetite reduction, insomnia, headache, diarrhea, nausea and abdominal pain (stomach ache). Since patients taking Libertek can lose weight, it is advisable that they weigh themselves regularly. Your doctor may stop treatment with Libertek if the patient loses too much weight. For the full list of all side effects reported with Libertek, see the Package Leaflet.
Libertek should not be used in people who may be hypersensitive (allergic) to roflumilast or any of the other substances. It must also not be used in patients with moderate or severe liver problems. Libertek is not recommended for patients with diseases that severely weaken the immune system (the body's natural defenses). Since there have been rare cases of patients receiving Libertek who developed suicidal thoughts, the medicine is also not recommended in patients who have suffered from depression with suicidal thoughts.
Why has Libertek - roflumilast been approved?
The CHMP noted that there was a need for new treatments for COPD and that the main studies showed a modest benefit of Libertek in patients with severe COPD. The benefit was evaluated in addition to the effects of the treatments to which the patients were already subjected. After assessing all available data on the effects of the medicine, the committee determined that Libertek's benefits are greater than its risks and recommended that it be given marketing authorization.
What measures are being taken to ensure the safe use of Libertek - roflumilast?
The company that makes Libertek will ensure that medical personnel who prescribe the medicine in all Member States of the European Union (EU) have material containing information on the side effects of the medicine and how it should be used. The company will also provide cards for patients indicating to them what information they should report to their doctor about their symptoms and previous illnesses, to help the doctor figure out if Libertek is right for them. The card will include a section where the patient can record his weight.
More information on Libertek - roflumilast
On 28 February 2011, the European Commission granted a marketing authorization for Libertek, valid throughout the European Union, to Nycomed GmbH. The marketing authorization is valid for five years, after which it can be renewed.
This authorization is based on the authorization granted to Daxas in 2010 ("informed consent").
The full EPAR for Libertek is available on the Agency's website. For more information about treatment with Libertek, read the package leaflet (also part of the EPAR) or contact your doctor or pharmacist.
Last update of this summary: 01-2011.