What is phenylketonuria
Phenylketonuria (PKU) is an autosomal recessive hereditary metabolic disorder that affects 1 individual per 10, 000, indiscriminately, whether we speak of white and black race, although it seems to occur more in homozygosity than heterozygotes.
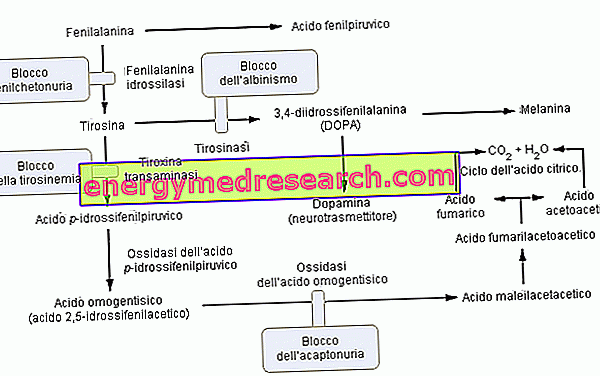
Belonging to the group of hyperphenylalaninemias, phenylketonuria significantly impairs the metabolism of phenylalanine and in particular its conversion to tyrosine; phenylketonuria is recognized by the high urinary levels of phenylalanine and some derivatives (phenylpyruvate, phenylacetate, phenylactate and phenylacetylglutamine).
The most serious complication of phenylketonuria is mental retardation.
Phenylalanine, tyrosine and derivatives
Phenylalanine is an essential amino acid and constitutes most of the dietary proteins; it can be converted by the enzyme phenylalanine hydroxylase into tyrosine (by adding a hydroxyl group -OH). In turn, tyrosine is a precursor amino acid for the synthesis of:
- L-DOPA (intermediate of dopamine synthesis)
- Epinephrine
- Norepinephrine (all neurotransmitters).
Phenylketonuria (PKU) mechanism
As anticipated, in phenylketonuria, due to one or more (6 in all) chromosomal mutations, the expression (therefore the metabolic activity) of phenylalanine hydroxylase is almost zero. These alterations can be of various kinds (from "missense" changes to "splicing" defects or even "partial deletions") but what matters is that for this enzymatic inefficiency the levels of blood phenylalanine (which are normally 1mg / 100ml) in DOMINANT phenylketonuria they easily reach quantities up to 50 times higher.
Function of the enzyme phenylalanine hydroxylase: To produce tyrosine (+ dihydrobiopterin), phenylalanine hydroxylase requires: phenylalanine, oxygen and tetrahydrobiopterin (a reduced pteridine that acts as a co-factor); the reaction is also reversible and dihydrobiopterin can be converted (thanks to the enzyme dihydropterin reductase ) into tetrahydrobiopterin.
Complications
Phenylketonuria can give rise to more or less severe complications based on the severity of the pathological manifestation and the timeliness of the diagnosis; being a hereditary disease, phenylketonuria is distinguished in:
- Dominant, therefore characterized by COMPLETE inactivity of the phenylalanine hydroxylase enzyme
- Recessive, in which only 30% of the total enzyme assets are active.
The complications of phenylketonuria are attributable, and directly proportional, to the metabolic accumulation of phenylalanine, its derivatives and the reduced synthesis of tyrosine. In the pathology, the excess phenylalanine is relatively effectively filtered by the kidney which reabsorbs it only partially eliminating it with the urine; however, the persistence of hyper-phenylalaninemia levels results in a metabolic reaction of molecular CONVERSION into phenylpyruvic acid and / or other derivatives that are easier to drain (phenylpyruvate, phenylacetate, phenylactate).
What complicates phenylketonuria is the toxicity of phenylalanine, phenylpiruvic acid and its derivatives against the central nervous system (CNS). These are soluble molecules in the cerebrospinal fluid, therefore able to go beyond the blood-brain barrier; unfortunately, their excessive presence during brain development inevitably leads to a form of mental retardation.
NB . The plasma concentrations of the other amino acids are mildly reduced, probably due to feedback to intestinal absorption or renal tubular reabsorption.
Brain damage, as a serious complication of phenylketonuria, is caused by the subtraction of other essential amino acids in proteosynthesis, in particular in the formation of polyribosomes, myelin, noradrenaline and serotonin. Phenylketonuria - not visible immediately after birth but after a few years - if left untreated, requires the child's hospitalization and is completely irreversible.
Phenylketonuria, in the advanced stage, can also be clearly visible to the naked eye; the high concentrations of phenylalanine, by inhibiting the enzyme tyrosinase, significantly compromise the synthesis of melanin by reducing the pigmentation of the skin and hair; moreover, the accumulation of phenylacetate in the hair and in the skin gives the phenylketonics a strong and unpleasant "mouse odor".
Differential diagnosis
Classical phenylketonuria and phenylketonuria due to enzyme tetrahydrobiopterin deficiency
Phenylketonuria does not have a single pathological picture; there is also a form of phenylketonuria induced by the deficiency of another enzyme: tetrahydrobiopterin . These subjects are recognizable from those affected by classical phenylketonuria since, following oral loads of tetrahydrobiopterin, they drastically reduce the levels of plasma phenylalanine in a very short time.
Fortunately, today it is possible to subject the newborn to an early diagnosis by blood chemistry analysis in order to limit most of the cognitive damage.
Phenylketonuria therapy
Being caused by the accumulation of phenylalanine, phenylpiruvic acid and other derivatives, phenylketonuria can be controlled more or less effectively by reducing the nutritional intake of phenylalanine. In case of positivity, phenylketonuria requires an almost absolute dietary restriction, in which all foods with a high protein content (in which phenylalanine is also present) are eliminated (or drastically limited); among these we remember:
- aspartame, spirulina algae, peanuts or peanut butter, dried fruit, highly-protein meat, cocoa, aged cheeses, high-protein fish (tuna), offal, game meat, legumes, soy flour, soy protein isolate.
The protein requirement is then covered through specific protein and amino acid supplements free of phenylalanine.
Bibliography:
- Genetic concepts - WS Klug, CA Spencer - Pearson - Page 375


