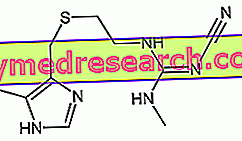
Generality
Hydrogen sulphide - otherwise known as hydrogen sulfide or hydrogen sulfide (H 2 S) - is a molecule soluble in water and ethanol, which gives off a very strong smell of "rotten eggs".

Hydrogen sulfide (H 2 S) is poisonous and even deadly to humans; its release in the air occurs mainly following:
- Bacterial or enzymatic decomposition of sulfur-containing proteins in stabilizing bonds, such as disulfide bridges and in sulfur amino acids
- Protein firing and secondary, tertiary and quaternary structure denaturation with disulfide bridging failure.
Hydrogen sulphide (H 2 S) is highly present in the fumes deriving from: air pockets in the subsoil, crude oil and areas with high putrescent activity (such as ponds, marshes and swamps); hydrogen sulfide is one of the aromatic components most present in faeces and intestinal gases, but its release in large quantities occurs mainly in the production cycles of the food industry, in water purification with sludge, in oil refining, etc.
NB . The hydrogen sulfide (H 2 S) reacts with silver and superficially creates a black patina of silver sulphide, visible on rings, earrings and necklaces after the thermal baths.
Toxicity
Hydrogen sulfide (H 2 S) is a poison that acts by inhibiting mitochondrial respiration, therefore its toxic action affects all the cells of the body that exploit aerobic metabolism (practically all of them, except the red blood cells); the most dangerous characteristic of hydrogen sulfide (H 2 S) at medium-high concentrations is its ability to inactivate the olfactory sensory perception, as the only alarm bell due to the presence of hydrogen sulfide (H 2 S) in the air. At low concentrations, on the other hand, hydrogen sulfide (H 2 S) produces mucosal irritation, hyperventilation and pulmonary edema, and prolonged exposure leads to chronic fatigue, loss of appetite, headache, cognitive and memory disorders.
Hydrogen sulphide (H 2 S) is already perceptible in concentrations of 0.0047 parts per million (from 50% of people), while 10ppm represents the lower limit of toxicity without the risk of damage to health following exposure of 8 consecutive hours; with levels equal to 1000ppm of hydrogen sulfide (H 2 S) there is an immediate collapse even after a single breath.
| SUMMARY TABLE OF THE TOXICITY OF SULFIDRIC ACID - SULFUR HYDROGEN - DIHYDROGEN SOLFUR (H2S) | |
Concentrations in parts per million (ppm) | Effect on the human organism |
0, 0047ppm | Lower perception limit for 50% of people |
<10ppm | Exposure limit without damage to health, for 8 hours a day |
10-20ppm | Limit beyond which the eyes are irritated by the gas |
50-100ppm | Concentration that causes cellular damage |
100-150ppm | Concentration that paralyzes the olfactory nerve |
320-530ppm | Concentration causing pulmonary edema |
530-1000ppm | Concentration that causes hyperventilation |
800ppm | Lower mortality limit of 50% of people after 5 minutes of exposure |
> 1000ppm | Minimum concentration that causes collapse by suffocation after 1 single breath |
Hydrogen sulfide in foods
Hydrogen sulfide (H 2 S) can be produced in foods from which it is released by gaseous evaporation; hydrogen sulfide (H 2 S) is a typical derivative of some chemical transformations such as, for example, disulphide bridge breakdown and hydrogenation aggregation (H +) following "protein firing" (denaturation). This reaction is clearly perceptible in the hard-boiled egg which, by producing hydrogen sulfide (H 2 S) starting from the sulfur of the albumen (which, although volatile, is retained by the shell), at the moment of shelling it instantly frees the gas making it perceptible smell. Recall also that, in the hard-boiled egg, hydrogen sulfide (H 2 S) released by the firing of the albumen is responsible for the iron (surface) chelation of the yolk with the production of a salt called ferrous sulfide (FeS), plus two hydrogenions; the chemical reaction is as follows:
H2S + Fe ++ → FeS + H 2
NB . Ferrous sulfide is a compound which, although chelating the yolk's iron and partially preventing its metabolic use, is harmless at low concentrations. However, it is not to be underestimated at high dosages.
Hydrogen sulphide (H 2 S) is also a compound that facilitates the distinction between healthy foodstuffs of animal origin and those in the process of decomposition; this happens due to the putrescent bacterial action both towards the sulphide bridges of the proteins and against the sulfur amino acids, with the consequent release of sulfur destined for the synthesis of hydrogen sulphide (H 2 S). NB . This process is recognizable above all in the degradation of eggs and fish damaged due to poor conservation.