Aztreonam is a beta-lactam type antibiotic belonging to the class of monobactams. It is a completely synthetic drug with a certain resistance against beta-lactamases (enzymes produced by some bacterial species able to hydrolyze the beta-lactam ring of the antibiotic, thus making it ineffective).
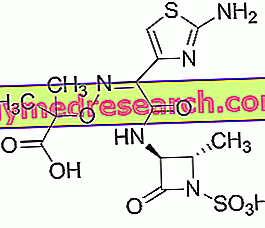
Aztreonam - Chemical Structure
The action spectrum of aztreonam is restricted to Gram-negative bacteria only.
Aztreonam is marketed in the form of pharmaceutical formulations suitable both for parenteral administration and for inhalation administration.
Indications
For what it uses
Aztreonam is used to treat infections caused by bacteria that are sensitive to it.
More precisely, aztreonam is indicated in the treatment of:
- Serious bacterial infections caused by Gram-negative bacteria;
- Gram-negative infections in weak and / or immunosuppressed patients;
- Pseudomonas aeruginosa chronic pulmonary infections in patients aged 6 years and over with cystic fibrosis (inhalation administration).
Furthermore, aztreonam can be used in preventive therapy of surgical infections.
Warnings
Patients with impaired hepatic and / or renal function should have regular follow-up for the duration of aztreonam treatment.
Before starting aztreonam treatment, you must inform your doctor if you are in any of the following conditions:
- If you are allergic to other types of antibiotics;
- If you have kidney problems;
- If you happened to expel blood with the cough;
- If the results of pulmonary function tests are altered.
Treatment with aztreonam could favor the development of Clostridium difficile infections (a Gram-positive bacterium) which is the main cause of pseudomembranous colitis. Colitis usually occurs with severe diarrhea and may require interruption of aztreonam therapy. Similarly, aztreonam treatment can promote superinfections with other Gram-positive bacteria or fungi normally found in human bacterial flora.
Aztreonam may cause a prolongation of prothrombin time.
Rare cases of seizures have been reported following aztreonam treatment.
Caution should be used in concomitant administration of aztreonam and other antibiotics.
Aztreonam can alter blood tests and Coombs test results.
Interactions
Aztreonam blood concentration may be increased by concomitant administration of valproate (a drug used in the treatment of epilepsy) or furosemide (a diuretic).
Aztreonam may increase the activity of oral anticoagulants, therefore - in patients on therapy with such drugs - regular checks should be performed. Furthermore, an adjustment of the administered anticoagulant dose may also be necessary.
In any case, it is necessary to inform the doctor if you are taking - or if you have recently been taken - drugs of any kind, including over-the-counter medicines and herbal and / or homeopathic products.
Side effects
Aztreonam can cause various adverse effects, although not all patients experience them. The type of adverse effects and the intensity with which they occur depend on the different sensitivity that each individual has towards the drug.
Listed below are the main side effects that may occur during aztreonam therapy, when given intravenously or intramuscularly.
Blood and lymphatic system disorders
Treatment with aztreonam may cause:
- Pancytopenia, that is the abnormal decrease of all blood cells;
- Increased number of platelets in the bloodstream;
- Leukocytosis, ie the increase in the number of leukocytes in the bloodstream;
- Eosinophilia, ie the increase in plasma concentration of eosinophils;
- Anemia;
- Plateletopenia (ie the decrease in the number of platelets in the bloodstream), with consequent increased risk of bleeding;
- Neutropenia, ie the reduction in the number of neutrophils in the bloodstream.
Gastrointestinal disorders
Aztreonam therapy can cause gastrointestinal bleeding, pseudomembranous colitis (caused by Clostridium difficile superinfections), abdominal pain, nausea, vomiting, diarrhea, halitosis and mouth ulceration.
Cardiovascular disorders
Treatment with aztreonam may cause:
- Increase in prothrombin time;
- Hypotension;
- Electrocardiogram changes;
- phlebitis;
- thrombophlebitis;
- Purple.
Skin and subcutaneous tissue disorders
Aztreonam therapy may cause:
- Skin rash;
- Urticaria;
- Itch;
- Angioedema;
- Erythema multiforme;
- petechiae;
- Toxic epidermal necrolysis;
- Exfoliative dermatitis;
- Hyperhidrosis.
Vaginal infections
Treatment with aztreonam may promote the onset of vaginitis and vaginal candidiasis (caused by superinfections with Candida albicans ).
Nervous system disorders
Aztreonam therapy may cause:
- dizziness;
- Headache;
- paresthesia;
- Dizziness;
- Convulsions.
Psychiatric disorders
Aztreonam can cause insomnia and induce confusion.
Lung and respiratory tract disorders
Treatment with aztreonam may cause:
- Dyspnoea;
- Wheezing breath;
- Sneezing;
- Nasal congestion;
- Bronchospasm.
Hepatobiliary disorders
Aztreonam therapy may cause increased blood concentration of transaminase and alkaline phosphatase, hepatitis and jaundice.
Other side effects
Other side effects that may occur during aztreonam treatment are:
- Allergic reactions in sensitive (rather rare) subjects;
- Temperature;
- Malaise;
- Asthenia;
- Myalgia;
- Alterations in the sense of taste;
- Diplopia (double view);
- Tinnitus, that is an auditory disorder characterized by the perception of rustling, buzzing, whistling, etc .;
- Breast discomfort;
- Discomfort at the injection site.
Side effects of aztreonam administered by inhalation
When administered by inhalation, aztreonam can induce side effects, such as:
- Cough;
- Wheezing breath;
- breathlessness;
- Sore throat;
- Closed nose or runny nose;
- Fever (especially in children);
- Difficulty breathing;
- Chest discomfort;
- Cough with blood expulsion;
- Skin eruptions;
- arthralgia;
- Joint swelling;
- Worsening of pulmonary function test results;
- Allergic reactions in sensitive subjects.
Overdose
In case of overdose with aztreonam (when given parenterally) hemodialysis and / or peritoneal dialysis may be useful.
In any case, if you suspect you have taken an overdose of medication - regardless of the route of administration - you must contact your doctor immediately or contact the nearest hospital.
Action mechanism
Aztreonam exerts its antibiotic action by interfering with the synthesis of the bacterial cell wall, ie it interferes with the synthesis of the peptidoglycan.
Peptidoglycan is a polymer made up of parallel chains of nitrogenated carbohydrates, joined together by transverse bonds between amino acid residues.
These bonds are formed thanks to the action of enzymes belonging to the peptidase family.
Aztreonam binds to some types of peptidase thus preventing the formation of the aforementioned transverse bonds. In this way, weak areas are created inside the peptidoglycan that lead to the lysis and death of the bacterial cell.
Mode of Use - Posology
Aztreonam is available for intravenous or intramuscular administration in the form of a powder and solvent for solution for injection which must be mixed just before their use.
Furthermore, the drug is also available for inhalation administration as a powder and solvent for nebulizer.
The type of administration, the dose of aztreonam and the duration of treatment must be established by the doctor depending on the type and severity of the infection to be treated and on the age, weight and condition of the patients.
The following are some indications on aztreonam doses usually used in therapy.
Intravenous or intramuscular administration
In adults, the dose of aztreonam usually used varies from 500 mg to 2 g of drug, to be administered every 6, 8 or 12 hours.
In children, the dose of drug usually used is 30 mg / kg of body weight, to be administered every 6-8 hours.
In children with a body weight over 40 kg the same doses used in adults are administered.
In the prophylaxis of surgical infections, usually, 1 g of aztreonam is administered just before the intervention and is then administered again after 8-16 hours from the first dose.
In patients with liver and / or kidney disease, the doctor may decide to reduce the doses of aztreonam usually administered.
Administration by inhalation
When administered by inhalation, it is recommended to take aztreonam three times a day, in repeated cycles of 28 days of therapy followed by 28 days off without taking the drug.
The three doses of aztreonam must be taken at a distance of at least four hours from each other by using the appropriate nebulizer.
The use of aztreonam by inhalation is not indicated in children under 6 years of age.
Pregnancy and breastfeeding
Aztreonam is able to cross the placenta and reach the fetus. Animal studies have shown no toxicity to the fetus following the use of aztreonam. However, as there are no reliable data on the safe use of aztreonam by pregnant women, the drug should only be administered to pregnant women if absolutely necessary.
Aztreonam is excreted in breast milk, therefore the possibility of interrupting breastfeeding should be considered before taking the drug. In any case, you should always seek advice from your doctor.
Contraindications
The use of aztreonam is contraindicated in patients with hypersensitivity known to aztreonam itself.



