By Dr. Rita Fabbri
Contraindications, special warnings and appropriate precautions for use, undesirable effects
Aloe vera gel can be used safely in topical applications: the range of these products available on the market is truly vast. Regarding Aloe vera juice, there is currently no precise data on the optimal daily dose, however it is recommended not to take more than 250 ml / day (38).
For topical use no known contraindication, no warning required and no reported side effects. Although rare, cases of allergic reactions have been reported.
It has also been shown that Aloe vera gel delays the healing of deep vertical surgical wounds, such as those produced during cesarean delivery (39).
For systemic use, see appendix below.
Pharmacological notes on anthraquinones
Anthraquinones are substances that stimulate intestinal peristalsis, therefore they have a laxative action.
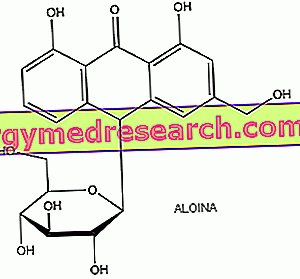
Anthraquinones have a general chemical structure characterized by three condensed benzene rings and by substituents which generally occupy positions 9 and 10 as they are particularly reactive. Anthraquinones are normally found in the form of glycosides, chemical compounds formed by a sugary part (called glycoon) and a non-sugary part (called aglycone). In anthraquinone glycosides, aglycones belong to the class of anthracene derivatives; barbaloin, for example, is a C-glycoside derived from aloe-emodin antrone. The glycosidic form allows these compounds to pass unchanged through the stomach and the small intestine up to the large intestine where they are transformed by the bacterial microflora into the respective aglycones, the true active metabolites, which perform the laxative activity locally in two ways : accumulation of fluid in the intestinal lumen and modification of intestinal motility; after which, without being absorbed, they bind to the intestinal contents and are expelled with the faeces.
The lack or reduced absorption of anthracene glycosides by the body, together with the absence of alterations towards the intestinal mucosa, makes these products safe and free from undesired effects, provided that some contraindications are respected and, very importantly, that they are used at the recommended doses and administered only in case of real need.
Stimulant laxatives are indicated in the short-term treatment of occasional constipation. In chronic constipation, on the other hand, the change in eating habits, physical activity and intestinal re-education represent the best solution (40-41).
The use of laxatives should be avoided for prolonged periods and it is advisable to consult a doctor if they are taken beyond two weeks.
When episodes of constipation occur repeatedly, it is advisable to investigate the causes of the disorder.
Constipation is not always associated with intestinal atony, sometimes it can be caused by hyperkinesia or dyskinesia as in the case of irritable bowel syndrome. Very often constipation is aggravated by nervous factors, anxiety or stress. In all these cases the anthraquinones are not recommended.
All stimulant laxatives are contraindicated in case of pregnancy (42-44) and breastfeeding (45) - small amounts of metabolites pass into breast milk - in children under 10 years of age, in acute inflammatory bowel diseases (ulcerative colitis, enterocolitis, appendicitis, Crohn's disease), in case of abdominal pain of unknown origin, in intestinal obstructions and stenosis and in the serious states of dehydration with lack of liquids and electrolytes (46).
Like all laxatives, anthraquinones should not be administered in the presence of an undiagnosed, acute or persistent abdominal symptomatology.
High doses of anthraquinone laxatives cause almost complete emptying of the colon and the natural lack of stimulation the next day (or even two days thereafter) can push patients to reuse the laxative, perhaps increasing the dosage; a psychological dependence is thus created, dictated by the subject's anxiety to regularize any delays between an evacuation and the following one.
The abuse of anthraquinone laxatives can cause disorders of water and electrolyte balance, mainly hypokalemia, atonic colon and aggravation of constipation.
Hypokalaemia enhances the action of cardiac glycosides and interacts with antiarrhythmic drugs. Combination with other drugs that induce hypokalemia (such as thiazine diuretics, corticosteroids) can aggravate electrolyte imbalance (47). The level of electrolytes, especially potassium, must always be monitored, particularly in elderly and young people.
The dark pigmentation of the colonic mucosa, defined as pseudomelanosis coli, observed following chronic intake of anthraquinone laxatives (but also of other laxatives) is not harmful and is reversible with treatment suspension.
Also the yellow-brown or red-violet color of the urine (pH dependent) due to the effect of anthraquinone metabolites is not clinically significant (48-49).
Abdominal spasms and pain can sometimes occur, especially in patients with an irritable colon. An observational study is very recent that shows how a colon-specific formulation containing anthraquinones from Senna angustifolia, mixed with microencapsulated oils of Mentha piperita and Matricaria camomilla, is able to counteract constipation without determining evident inflammatory states characterized by pain, spasms, distension abdominal, meteorism, flatulence and diarrheal states (50).
Bibliography
- Kanter, MM, Free radicals and exercise: effects of nutritional antioxidant supplementation. Exerc. Sport Sci. Rev., 23: 375, 1995.
- Kanter, MM, et al., Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. 74: 965.1993.
- Yamaguchi et Al. (1993) Components of the gel of Aloe vera. Bioscience biotechnology and biochemistry. 57-8.1350-1352.
- Saben-Farideh (1993) Studies of the status of antioxidant enzymes and metabolites fallow burn injury, and the presence of antioxidant enzymes in the Aloe vera plant (tumor necrosis factor, glutathione), p 138.
- Davis, Didonato, Hartman, (1994). Anti-inflammatory and wound healing activity of a growth substance in Aloe vera.
- PubMed January 1989. Davis, Maro.
- Lushbaugh CC and Hale DB: Experimental acute radiodermatitis following beta radiation. V. Histopathological study of the mode of action of therapy with Aloe vera. Cancer 6, 690-698, 1953.
- Beneficial Effects of Aloe in Wound Healing Heggers JP, Pelley RP, Robson MC Phytotherapy Research, vol 7, S48-S52 (1993). Department of Surgery and Graduate School of Biomedical Sciences, University of Texas Medical Branch, Galveston, USA.
- Davis RH, Leitner MG, and Russo JM: Aloe vera, a natural approach for treating wounds, edema, and pain in diabetes. J Am Pod Med Assoc 78, 60-68, 1988.
- Ajabnoor MA: Effect of a loes on blood glucose levels in normal and alloxan diabetic mice. J.Ethnopharmacol 28, 215-220, 1990
- El Zawahry M, Hegazy MR, and Helal M: Use of aloe in treating leg ulcers and dermatoses . Int J Dermatol 12, 68-73, 1973.
- Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo-controlled, double-blind study Trop Med Int Health 1996 Aug; 1 (4): 505-9 Syed TA; Ahmad SA; Holt AH; Ahmad SA, Ahmad SH; Afzal M Department of Clinical Physiology, Malmo University Hospital, Sweden.
- Basic Medicine Service. Social Security Institute. Rep. San Marino. January, 2000 Andriani, Bugli, Alders, Castelli, et al.
- Grindlay D and Reynolds T: The Aloe vera leaf phenomena: A review of the properties and modem use of the leaf parenchyma gel. J Ethnopharmacol 16, 117-151, 1986.
- Shelton RW: Aloe vera, its chemical and therapeutic properties . Int. J Dermatol 30, 679-683, 1991.
- Kahlon JB, et al .: In vitro evaluation of the synergistic antiviral effects of acemannan in combination with azidothymidine and acyclorir. Mol Biother 3, 214-223, 1991.
- Anonymous: Aloe vera may boost AZT. Med Tribune, August 22, 1991, p.4.
- Pulse TL and Uhlig E: A significant improvement in a clinical pilot study using nutritional supplements, essential fatty acids and stabilized Aloe vera juice in 29 seropositive, ARC and AIDS patients. J Adv Med 3, 209-230, 1990.
- Singer J: A randomized placebo-controlled trial of oral acemannan as an adjunctive to anti-retroviral therapy in advanced HIV disease. Int Conf AIDS 9 (1), 494, 1993. [Abstract No. PO-B28-2153]
- Sheets MA, et al .: Studies of the effect of acemannan on retrovirus infections: Clinical stabilization of feline leukemia virus-infected cats. Mol Biother 3, 41-45, 1991.
- Hart LA, et al .: Effects of low molecular weight constituents from Aloe vera gel on oxidative metabolism and cytotoxic and bactericidal activities of human neutrophils . Int J Immunol Pharmacol 12, 427-434, 1990.
- Womble D and Helderman JH: Enhancement of all sustainability of human lymphocytes by acemannan (CarrisynTM). Int J Immunopharmacol 10, 967-974, 1988.
- Peng SY, et al .: Decreased mortality of Norman murine sarcoma in mice treated with the immunomodulator, acemannan. Mol Biother 3, 79-87, 1991.
- Harris C, et al .: Efficacy of acemannan in treatment of canine and feline spontaneous neoplasms. Mol Biother 3, 207-213, 1991.
- USSR cancer research laboratories. 1986. Gribel, Pashinskii.
- Fujita K, Ito S, Teradaira R, Beppu H, Properties of carboxypeptidase from Aloe, Biochem. Pharmacol., 28: 1261-1262, 1979.
- Fujita K, Teradaira R, Nagatsu T: Bradykininase activity of Aloe extract, Biochem. Pharmacol., 25: 205, 1976.
- Davis RH, et al .: Anti-inflammatory activity of Aloe vera against a spectrum of irritants. J Am Pod Med Assoc 79, 263-266, 1989.
- Davis RH, et al .: The isolation of an active inhibitory system from an extract of Aloe vera. J Am Pod Med Assoc 1991 May; 81 (5): 258-61.
- Saito H, Purification of Active Substances of Aloe a. and their Biological and pharmacological activity, Phytother. Res., 7: S14-S1, 1993.
- Davis RH, et al .: Aloe vera, hydrocortisone, and sterol influence on wound tensile strength and anti-inflammation. J Am Pod Med Assoc 1994 Dec; 84 (12): 614-21.
- Davis RH, et al .: Topical effect of Aloe with ribonucleic acid and vitamin C on adjuvant arthritis. J Am Pod Med Assoc 75, 229-237, 1985.
- Bland J: Effect of orally-consumed Aloe vera juice on human gastrointestinal function. Natural Foods Network Newslett, August, 1985.
- Blitz JJ, Smith JW, and Gerard JR: Aloe vera gel in peptic ulcer therapy: Preliminary report. J Am Osteopathol Soc 62, 731-735, 1963.
- Yamaguchi I, Mega N, Sanada H: Components of the gel of Aloe vera Burm-f, Biosci. Biotech. Biochem., 57 (8): 1350-1352, 1993.
- Shida T, et al .: Effect of Aloe extract on peripheral phagocytosis in adult bronchial asthma. Planta Medica 51, 273-275, 1985.
- Godding EW: Therapeutics of laxative agents with special reference to anthraquinones. Pharmacology 14 (Suppl 1), 78-101, 1976.
- Strengths and Limitations of Aloe vera. Rowan Hamilton American Journal of Natural Medicine, Vol 5, No. 10; 30-33, Dec. 1998.
- Schmidt JM and Greenspoon JS: Aloe vera dermal wound gel is associated with a delay in wound healing. Obstet Gynecol 78, 115-117, 1991.
- Steinegger E, Hansel R. Aloe. In: Pharmakognosie, 5th ed. Berlin Springer, 1992: 428-31.
- Muller-Lissner S. Adverse effects of laxatives: fact and fiction. Pharmacology 1993.47 (Suppl1): 138-45.
- Westendorf J. Anthranoid Derivatives - Aloe Species. In: De Smet PAGM, Keller K, Hansel R, Chandler RF, editors. Adverse Effects of Herbal Drugs, Volume 2. Berlin: Springer, 1993: 119-23.
- Bangel E, Pospisil M, Roetz R, Falk W. Tierexperimentelle pharmakologische Untersuchungen zur Frage der abortiven und teratogenen Wirkung sowie zur Hyperamie von Aloe. Steiner-Informationsdienst 1975; 4: 1-25.
- Schmidt L. Vergleichende Pharmakologie und Toxikologie der Laxantien. Arch Exper Path Phamakol 1995; 226: 207-18.
- Faber P, Strenge-Hesse A. Revelance of rhein excretion into breast milk. Pharmacology 1988; 36 (Suppl 1): 212-20.
- Reynolds JEF, editor. Martindale - The Extra Phama-copoeia. 31st ed. London: Royal Pharmaceutical Society, 1996: 1202-3, 1240-1.
- Brunton LL. Agents affecting gastrointestinal water flux and motility, emesis and antiemetics; bile acids and pancreatic enzymes In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 9th ed. New York: McGrew-Hill, 1996: 917-36.
- German FJ. Laxative use in constipation. Am J Gastroenterol 1985; 80: 303-9.
- Ewe K, Karbach U. Factitious diarrhoea. Clin Gastroenterol 1986; 15: 723-40.
- Di Pierro F, Rapacioli G, Callegari A, Attolico M, Ivaldi L, Candidi C. Clinical efficacy in the constipation of a preparation based on anthraquinones and essential oils: concomitant laxative effect with anti-inflammatory action. The Gastroenterologist ; Year XXXI, n.1-2 / 2009.



