SUSPIRIA ® is a drug based on Cetirizine dichloridate
THERAPEUTIC GROUP: Antihistamines for systemic use - H1 antagonist
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Directions SUSPIRIA ® - Cetirizine
SUSPIRIA ® is used in the clinical setting in the treatment of the characteristic symptoms present in the course of allergic rhinitis and chronic idiopathic urticaria.
Mechanism of action SUSPIRIA ® - Cetirizine
Cetirizine, the active ingredient of SUSPIRIA ®, is a highly selective h1 histaminergic receptor antagonist whose activation is partly responsible for the typical respiratory and skin symptoms present during allergic reactions.
More precisely, the activation of these receptors, supported by phlogistic mediators such as histamine, determines repeated contractions responsible for bronchospasm at the level of the bronchial smooth muscle, and at the level of the perivenular capillaries a significant increase in permeability with edema and congestion of the respiratory mucosa, and consequent reduction of ventilation capacity.
Cetirizine, interacting with high affinity with these receptors, inhibits its activation thus controlling the onset of the aforementioned biological effects and guaranteeing in a few minutes from the administration a prompt regression of the symptomatology.
The long half-life of this active ingredient also allows the drug to persist in circulation for several hours, significantly simplifying its intake and limiting therapy to once-daily administration.
After its activity, Cetirizine is largely eliminated unchanged through the urine.
Studies carried out and clinical efficacy
CETIRIZINE IN THE CONTROL OF THE PRODUCTION OF INFLAMMATORY CYTOKIN
Eur Ann Allergy Clin Immunol. 2004 Jun; 36 (6): 237-40.
Work demonstrating how the use of Cetirizine can significantly reduce the production of inflammatory cytokines in patients with perennial allergic rhinitis, thus also controlling the recruitment of inflammatory cells.
THE EFFECTIVENESS OF CETIRIZINE IN THE TREATMENT OF ALLERGIC RHINITIS COMPLICATED BY ASMA
J Allergy Clin Immunol. 1995 May; 95 (5 Pt 1): 923-32.
Study demonstrating how the daily intake of Cetirizine 10 mg can be effective and safe in reducing the typical allergic symptoms affecting the upper respiratory tract in patients with allergic rhinitis and concomitant asthma.
AUTOMATIC HEPATITIS FROM CETIRIZINA
Ann Pharmacother. 2004 Nov; 38 (11): 1844-7. Epub 2004 Sep 21.
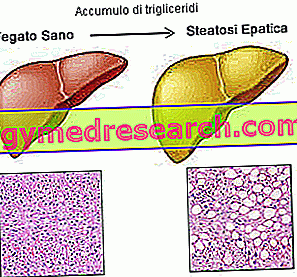
An extremely interesting Italian study that points out that, although Cetirizine is a drug with low hepatotoxic potential, it would be advisable to always consider the risk of the onset of autoimmune hepatitis associated with the use of this drug.
Method of use and dosage
SUSPIRIA ®
Tablets coated with 10 mg of Cetirizine dichloridate;
Oral drops of 10 mg of Cetirizine dihydrochloride per ml of solution.
The dosing schedule useful for the treatment of allergic manifestations, both respiratory and cutaneous, should be defined by the doctor on the basis of the patient's general health conditions, his age and the severity of the present clinical picture.
It is also important to consider the possibility of correcting the dosages normally used in clinical settings, for patients suffering from kidney disease or severe liver disease.
Warnings SUSPIRIA ® - Cetirizine
The use of SUSPIRIA ® should be preceded by a careful medical examination in order to assess the prescriptive appropriateness and the possible presence of pathologies or conditions incompatible with the therapy itself.
In this regard, particular caution should be reserved for patients suffering from severe liver disease and nephropathy, in the usual consumers of alcohol and in patients with serious cardiac and neurological diseases.
SUSPIRIA ® in tablets contains lactose therefore its intake is generally not indicated in patients with lactase enzyme deficiency, glucose-galactose malabsorption syndrome, galactose intolerance.
SUSPIRIA ® in drops contains parahydroxybenzoates, excipients with a strong allergic activity.
Keep the medicine out of the reach of children, taking care to avoid using SUSPIRIA ® in children under 6 years of age.
PREGNANCY AND BREASTFEEDING
Given the absence of clinical data capable of fully characterizing the safety profile of Cetirizine for fetal health, it would be preferable to avoid taking SUSPIRIA ® during pregnancy and in the subsequent breastfeeding period.
Interactions
In order to preserve the pharmacokinetic and pharmacodynamic characteristics of Cetirizine, the simultaneous intake of alcohol and potentially nephrotoxic active ingredients should be avoided.
Contraindications SUSPIRIA ® - Cetirizine
The use of SUSPIRIA ® is contraindicated in case of hypersensitivity to the active ingredient or to one of its excipients or to other structurally related molecules, in patients suffering from severe liver disease and nephropathy and in small patients below 2 years.
Undesirable effects - Side effects
The use of SUSPIRIA ® especially when prolonged for a long time could determine the appearance of adverse reactions nausea, diarrhea, changes in liver function, fatigue, weakness, dizziness, itching and rash.
The side effects at the neurological level with headache, dizziness, sleepiness, nervousness and insomnia are more rare.
Note
SUSPIRIA ® is a drug subject to mandatory medical prescription.