Aloperidol is an antipsychotic drug belonging to the class of butyrophenones, of which it is the progenitor.
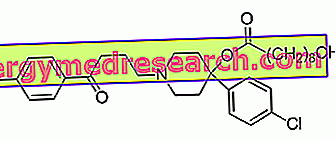
Haloperidol - Chemical Structure
It was synthesized in the 1950s by researcher Paul Janssen and was introduced to Europe for the treatment of psychoses in 1958.
It has a high antipsychotic and sedative power and also has antiemetic activity.
Most likely, haloperidol is best known under the trade names Haldol® and Serenase®.
Indications
For what it uses
Haloperidol is indicated for the treatment of:
- Acute and chronic schizophrenia;
- Acute mental confusion;
- Acute delusional and / or hallucinatory psychosis;
- Chronic psychosis;
- Paranoia;
- Ipocondriasi;
- Paranoid, schizoid, schizotypal, antisocial and some borderline type personality disorders;
- Choreiform movements (rapid, irregular and involuntary movements);
- Agitation and aggression in elderly patients;
- Tics and stuttering;
- He retched;
- Hiccup;
- Alcohol withdrawal syndrome;
- Treatment of intense pain, usually in association with opioid analgesics.
Aloperidol can also be used for the treatment of psychomotor agitation in the event of:
- Manic states;
- Dementia;
- Psychopathy;
- Acute and chronic schizophrenia;
- Oligophrenia (state of mental insufficiency, in general, is congenital or acquired during the first years of life);
- Alcoholism;
- Compulsive, paranoid or histrionic personality disorders.
Warnings
Haloperidol should be administered under the strict supervision of a psychiatrist.
Some cases of sudden death have been reported in psychiatric patients treated with haloperidol.
Aloperidol should not be administered intravenously, as - if administered by this route - there is an increased risk of prolongation of the QT interval (the interval of time required for the ventricular myocardium to depolarize and repolarize).
Caution should be used in the administration of haloperidol in patients suffering from cardiovascular diseases or who have a family history of prolongation of the QT interval. It is advisable to perform electrocardiogram checks before and during drug therapy.
It is also advised to perform periodic checks to determine the electrolyte rate.
The use of haloperidol by patients with dementia may increase the risk of cerebrovascular events.
Since haloperidol can cause clots, caution should be used when administering the drug in patients with a history of thrombus formation.
Aloperidol should be used with caution in elderly and depressed patients.
Epileptic patients - or with a history of seizure disorders - should use haloperidol with caution.
Attention should be paid to the administration of haloperidol during the manic phase of cyclical psychosis, due to the possibility of a rapid change in mood towards depression.
Attention should be paid to the administration of haloperidol in patients with liver disease.
Abrupt discontinuation of haloperidol therapy is not recommended, as withdrawal symptoms may occur or psychotic relapse may occur.
Haloperidol should not be used on its own in those patients in whom depression is dominant.
Aloperidol can cause the onset of neuroleptic malignant syndrome, in which case the treatment must be immediately suspended.
Haloperidol can cause sedation and decreased attention, therefore driving vehicles and / or using machines is not recommended.
Interactions
The use of haloperidol should be avoided in conjunction with the administration of drugs that prolong the QT interval. Some of these drugs are:
- Antiarrhythmics, such as quinidine, procainamide and amiodarone ;
- Some antihistamines ;
- Some antipsychotics ;
- Some antimalarials, such as quinidine and mefloquine ;
- Moxifloxacin, an antibacterial;
- Some antidepressants, such as paroxetine ;
- Ketoconazole, an antifungal drug.
Haloperidol should not be administered concomitantly with drugs capable of altering electrolyte concentrations. Concomitant use of diuretics (especially those that cause hypokalemia, ie a decrease in potassium in the bloodstream) should therefore be avoided.
The haloperidol plasma concentration may be increased by the co-administration of:
- Itraconazole, an antifungal;
- Buspirone and alprazolam, anxiolytic drugs;
- Nefazodone, venlafaxine, fluvoxamine, fluoxetine and sertraline, antidepressant drugs;
- Quinidine ;
- Chlorpromazine, an antipsychotic;
- Promethazine, an antihistamine drug.
Aloperidol may increase the central nervous system (CNS) depression induced by drugs, such as hypnotics, sedatives and strong analgesics ; it can also enhance the sedative effect of alcohol .
Haloperidol may decrease the therapeutic effects of levodopa (an anti-Parkinsonian drug).
Aloperidol decreases the metabolism of TCA (tricyclic antidepressants) thus increasing its plasma concentration.
The haloperidol plasma levels can be reduced by concomitant administration of carbamazepine, phenobarbital (anticonvulsant drugs) and rifampicin (an antibiotic).
Caution should be exercised in the co-administration of haloperidol and lithium (a drug used to treat bipolar disorders) due to the adverse effects that may occur.
Aloperidol can antagonize the effect of adrenaline, antihypertensives and fenindione (an oral anticoagulant).
Thyroxine may increase the toxicity of haloperidol.
Side effects
Haloperidol can trigger various side effects, although not all patients experience them. The type of adverse effects and the intensity with which they occur depend on each individual's sensitivity to the drug.
The following are the main side effects that may occur following haloperidol treatment.
Nervous system disorders
Haloperidol therapy may cause:
- Extrapyramidal disorders (Parkinson-like symptoms);
- agitation;
- hyperkinesia;
- hypokinesia;
- bradykinesia;
- Dyskinesia and tardive dyskinesia;
- Hypertonia;
- Dystonia;
- Motor dysfunctions;
- Tremors;
- Involuntary muscle contractions;
- Akathisia (inability to remain still);
- Drowsiness;
- Sedation;
- Dizziness;
- Headache;
- nystagmus;
- Convulsions.
Malignant Neuroleptic Syndrome
Neuroleptic Malignant Syndrome is a neurological disorder characterized by:
- Temperature;
- Dehydration;
- Muscle stiffness;
- akinesia;
- Sweating;
- Tachycardia;
- Arrhythmia;
- Changes in the state of consciousness that can progress to stupor and coma.
If these symptoms appear, haloperidol therapy should be stopped immediately and the doctor should be contacted immediately.
Psychiatric disorders
Haloperidol may cause decreased or lost libido, psychotic disorders, confusion, depression or insomnia.
Reproductive system and breast disorders
Haloperidol treatment can cause sexual dysfunction, amenorrhea (absence of menstrual cycle), sensation of discomfort or pain in the breast, dysmenorrhea (painful menstruation), menorrhagia (excessive blood loss during the menstrual cycle), galactorrhea (abnormal secretion of milk both in women than men), priapism (long and painful erection not accompanied by sexual excitement), gynecomastia (development of breasts in men).
Endocrine disorders
The use of haloperidol can cause hyperprolactinaemia (increased concentration of the hormone prolactin in the bloodstream) and trigger the syndrome of inappropriate secretion of the antidiuretic hormone (SIADH).
Cardiac disorders
Haloperidol treatment can give rise to tachycardia, ventricular fibrillation and extrasystoles.
Vascular pathologies
Haloperidol therapy can cause hypotension and orthostatic hypotension (ie a sharp drop in blood pressure when moving from a sitting or lying position to an upright position). In addition, the drug can promote thrombus formation.
Eye disorders
Haloperidol can cause vision disorders, blurred vision and oculogyric crises (rotary movements of the eyeball).
Gastrointestinal disorders
Haloperidol may cause nausea, vomiting, salivary hypersecretion, constipation and dry mouth.
Blood and lymphatic system disorders
Haloperidol treatment can cause disorders in the system responsible for blood cell production. These disorders cause a decrease in blood levels of white blood cells and platelets (respectively, leukopenia and thrombocytopenia).
Respiratory disorders
Haloperidol therapy can cause dyspnoea, bronchospasm, laryngospasm and laryngeal edema.
Hepatobiliary disorders
Haloperidol treatment can give rise to acute liver failure, hepatitis, cholestasis and jaundice.
Skin and subcutaneous tissue disorders
Aloperidol can cause photosensitivity reactions, hives, skin rash, pruritus, exfoliative dermatitis and hyperhidrosis.
Other side effects
Other side effects that may occur following haloperidol intake are:
- Allergic reactions in sensitive subjects;
- Weight gain or loss;
- Hypoglycemia;
- Hyponatremia (decrease in blood concentration of sodium);
- Edema;
- Gait disorders;
- Stiff neck;
- Muscle stiffness;
- spasms;
- lockjaw;
- Temperature;
- Unexpected death.
Overdose
There is no specific antidote for haloperidol overdose. The administration of activated charcoal may be useful.
The symptoms that can occur consist of an exacerbation of the side effects.
In any case, if you suspect you have taken an overdose of medication, you must immediately notify your doctor or go to the nearest hospital.
Action mechanism
Haloperidol is able to perform its antipsychotic action thanks to its antagonism towards D2 receptors of dopamine (DA) and 5-HT2 receptors of serotonin (5-HT). Indeed, these two endogenous monoamines are implicated in the etiology of psychiatric disorders.
Mode of Use - Posology
Aloperidol is available both for oral administration in the form of tablets and oral drops, and in vials for intramuscular administration.
The haloperidol dosage must be established by the doctor on a strictly individual basis.
The following are some indications on the doses of medication usually used depending on the use made of them.
In elderly patients a reduction in the administered dose may be necessary.
As a neuroleptic
In the acute phase, the usual dose is 5 mg of haloperidol intramuscularly to be repeated every hour until adequate symptom control is achieved. However, do not exceed 20 mg of drug per day. For oral administration, however, the dose is 2-20 mg / day, to be taken as a single dose or in divided doses.
In the chronic phase, the usual haloperidol dose is 1-3 mg, to be administered orally two or three times a day.
Control of psychomotor agitation
In the acute phase, the usual dose of haloperidol is 5 mg intramuscularly to be repeated every hour until symptom control is achieved. In any case, do not exceed 20 mg per day.
In the chronic phase haloperidol is usually administered orally, the dose is 0.5-1 mg up to a maximum of 2-3 mg, to be taken three times a day.
As a hypnotic
The usual dose is 2-3 mg of haloperidol to be taken orally in a single dose, the evening before bedtime.
As an antiemetic
Aloperidol can be used as an antiemetic for the treatment of vomiting of central origin at a dose of 5 mg of drug, to be administered intramuscularly.
Furthermore, the drug can be used in the prophylaxis of postoperative vomiting at a dose of 2.5-5 mg, to be administered intramuscularly at the end of the operation.
Pregnancy and breastfeeding
Use of haloperidol during pregnancy and lactation is not recommended due to the side effects that may occur in the newborn.
Contraindications
The use of haloperidol is contraindicated in the following cases:
- Known hypersensitivity to haloperidol;
- In patients in a comatose state;
- In strongly depressed patients with alcohol or other CNS active substances;
- In patients suffering from endogenous depression without agitation;
- In patients with Parkinson's disease;
- In patients with injuries to the basal ganglia;
- In patients with heart disease and / or QT interval prolongation;
- In patients with incorrect hypokalemia;
- In children;
- In pregnancy, ascertained or presumed;
- During breastfeeding.
Haloperidol decanoate
Aloperidol decanoate is a derivative of haloperidol introduced as a delay formulation for psychosis maintenance treatment.
The drug is injected intramuscularly every 4-6 weeks, in this way you get the same therapeutic efficacy that you would get with the daily administration of haloperidol orally.
The dosage administered is strictly individual and must be established by the physician according to the severity of the disease and according to the oral dose of haloperidol which was necessary for the maintenance of the patient before starting treatment with haloperidol decanoate.
In any case, it is recommended that the initial dose of haloperidol decanoate corresponds to 10-15 times the previous oral daily dose of haloperidol.