What is Methylthioninium chloride Proveblue - methylthioninium chloride?
Methylthioninium chloride Proveblue is a solution for injection containing the active substance methylthioninium chloride (5 mg / ml).
Methylthioninium chloride Proveblue is a "generic hybrid drug". This means that it is similar to a reference medicine, contains the same active ingredient, but in a different concentration. The reference medicine for Methylthioninium chloride Proveblue is Methylthioninium chloride, USP injection 1% p / v.
What is Methylthioninium chloride Proveblue used for - methylthioninium chloride?
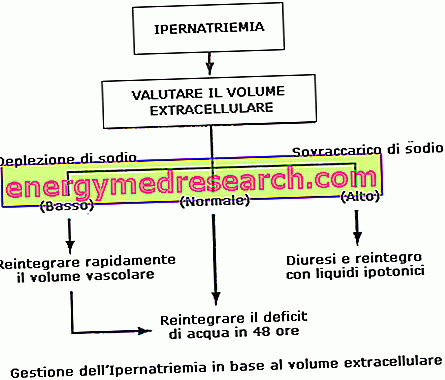
Methylthioninium chloride Proveblue is used in adults and children of all ages as an antidote for the symptomatic treatment of methaemoglobinaemia caused by exposure to drugs or chemicals.
Methemoglobinemia is a disease characterized by an accumulation in the blood of an altered form of hemoglobin that is not able to carry oxygen effectively. The substances that can cause methemoglobinemia include some antibiotics, local anesthetics, nitrates in drinking water and pesticides.
The medicine can only be obtained with a prescription.
How is Methylthioninium chloride Proveblue - methylthioninium chloride used?
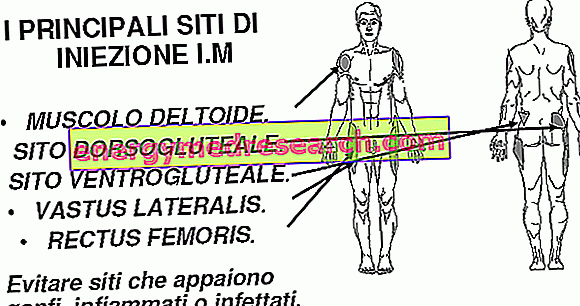
Methylthioninium chloride Proveblue is slowly injected into a vein over five minutes. It must be administered by a healthcare professional.
The usual dose for adults and children over three months of age is 1-2 mg per kilogram of body weight. A repeated dose may be given one hour after the first dose if symptoms persist or recur or if blood methaemoglobin levels remain higher than normal.
The dose in children aged three months or less is 0.3-0.5 mg / kg. You can be given a repeated dose after one hour.
How does Methylthioninium chloride Proveblue - methylthioninium chloride work?
To transport oxygen into the blood, hemoglobin must contain an iron atom in the "ferrous" form (Fe2 +). Exposure to certain drugs or chemical agents can transform hemoglobin iron into "ferric" (Fe3 +), typical of methemoglobinemia.
The active ingredient of Methylthioninium chloride Proveblue, methylthioninium chloride (also called methylene blue) helps to accelerate the conversion of altered hemoglobin to normal hemoglobin. This is because it accepts negatively charged electrons through an enzyme called "NADPH-methaemoglobin reductase". The electrons are then transferred to the iron atoms of the altered hemoglobin and convert them into the normal ferrous form.
How has Methylthioninium chloride Proveblue - methylthioninium chloride been studied?
Since methylthioninium chloride has been used in the European Union for several decades for the treatment of methaemoglobinaemia, the pharmaceutical company presented data on the use of methylthioninium chloride derived from the published literature.
What benefit has Methylthioninium chloride Proveblue - methylthioninium chloride shown during the studies?
The published literature has confirmed the efficacy of methylthioninium chloride in the treatment of methemoglobinemia caused by exposure to drugs or chemical agents in adults and children.
What are the risks associated with Methylthioninium chloride Proveblue - methylthioninium chloride?
The most frequent side effects reported with methylthioninium chloride are nausea, abdominal and thoracic pain, headache, dizziness, tremors, anxiety, confusion, dyspnoea (difficulty breathing), tachycardia (rapid heart beat), hypertension (high blood pressure), hyperhidrosis (excessive sweating) and the formation of methemoglobinemia. For the full list of all side effects reported with methylthioninium chloride, see the Package Leaflet.
Methylthioninium chloride Proveblue should not be used in case of hypersensitivity (allergy) to methylthioninium chloride or to any other thiazine dye (a group to which methylthioninium chloride belongs). The medicine is contraindicated in patients with:
- glucose-6-phosphate dehydrogenase deficiency (G6PD),
- methemoglobinemia caused by sodium nitrite,
- methemoglobinemia caused by chlorate poisoning,
- NADPH reductase deficiency.
Why has Methylthioninium chloride Proveblue - methylthioninium chloride been approved?
The Committee concluded that the long experience with the active ingredient, methylthioninium chloride, highlights its effectiveness in the treatment of methaemoglobinaemia. The CHMP therefore decided that the medicine's benefits are greater than its risks and recommended that it be given marketing authorization.
Other information about Methylthioninium chloride Proveblue - methylthioninium chloride
On May 6, 2011, the European Commission issued a marketing authorization to PROVEPHARM SAS for Methylthioninium chloride Proveblue, valid throughout the European Union. The marketing authorization is valid for five years, after which it can be renewed.
For more information about treatment with Methylthioninium chloride Proveblue, read the package leaflet (also part of the EPAR) or contact your doctor or pharmacist.
Last update of this summary: 03-2011.