BRUFEN ® is a drug based on Ibuprofen
THERAPEUTIC GROUP: Non-steroidal anti-inflammatory and antirheumatic drugs
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications BRUFEN ® Ibuprofen
BRUFEN ® is indicated both in the symptomatic treatment of rheumatological diseases including juvenile arthritis and in the treatment of inflammatory and painful states associated with musculoskeletal disorders, dysmenorrhea, surgical operations, migraine headaches and traumas.
Mechanism of action BRUFEN ® Ibuprofen
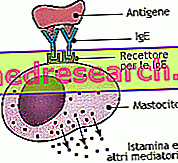
BRUFEN ® is a medicine based on ibuprofen, an active ingredient belonging to the family of non-steroidal anti-inflammatory drugs characterized by an impressive analgesic, anti-inflammatory and antipyretic activity.
Its therapeutic effect is guaranteed by the inhibitory action against prostaglandins, exercised through the enzymatic inhibition of cyclooxygenases, enzymes involved in the metabolism of arachidonic acid, therefore in the synthesis of molecules with pro-inflammatory activity.
Furthermore the inhibition of this metabolic pathway allows to shift the balance towards the synthesis of lipoxins, molecules able to inhibit adhesion and leukocyte chemotaxis, actively contrasting the inflammatory process.
In addition to its anti-inflammatory effect, which is particularly important in the treatment of rheumatic and skeletal muscle diseases, ibuprofen is also responsible for the analgesic action that allows the extension of therapeutic indications also to headache and migraine, and antipyretic so much to be considered as the second-line drug after paracetamol, also preferable over salicylates.
From the pharmacokinetic point of view, after oral administration, ibuprofen is absorbed in the gastrointestinal tract reaching maximum plasma concentrations after about 45 minutes and distributed to various tissues, especially at the level of the synovies, where it performs its therapeutic effect,
After a half-life of about 3 hours and a predominantly hepatic metabolism, the ibuprofen catabolites are excreted through the urine.
Studies carried out and clinical efficacy
1. THE IBUPROPHENE IN ADVANCED EXPERIMENTATION
Restor Neurol Neurosci. 2012 Jan 1; 30 (1): 9-19.
Very interesting experimental study that demonstrates how the anti-inflammatory action of ibuprofen can reduce lesions related to traumatic events in the brain, and favor the possible migration and engraftment of transplanted stem cells.
2. IBUPROFENE AND SURFACE TROMBOFLEBITE
J Thromb Haemost. 2012 Feb 23.
Work demonstrating the efficacy of ibuprofen, even when compared to the heparin delta, in reducing pain, the incidence of bleeding and the extension of the thrombus in over 70 patients suffering from superficial thrombophlebitis at risk of deep vein thrombosis.
3. IBUPROFENE, PSYCHOLOGY AND PAIN
Eur J Pain. 2012 Jan 19.
Recent study that shows how the analgesic action of ibuprofen can be significantly modulated by psychological factors. This work highlights how the psychological and neuroendocrine aspect associated can enhance or depress the biological effects of painkillers and anti-inflammatories including ibuprofen.
Method of use and dosage
BRUFEN ®
400 mg and 600 mg ibuprofen coated tablets;
Granules for oral solution of 600 mg of ibuprofen;
Suppositories of 600 mg of ibuprofen;
10% Ibuprofen cream (10 g of active ingredient in 100 g of cream).
The dosage to be used, although limited to the maximum dose of 1800 mg daily, varies considerably from patient to patient based on the physical characteristics and the severity of the clinical picture.
Dosage adaptation is inevitably required in elderly patients or those with renal insufficiency.
In any case, it would be advisable to use the minimum effective dose, able to improve the symptoms.
Warnings BRUFEN ® Ibuprofen
Treatment with BRUFEN ® should be supervised by your doctor in order to make the therapy effective and safe.
Particular attention should be paid to patients with reduced liver and kidney function, for whom periodic monitoring of some blood chemistry parameters is necessary.
The same caution is also required for patients with severe gastro-intestinal disorders due to the irritant action of ibuprofen on the mucous membranes.
Once the epidemiological data are known, showing an increased risk of cardiac, vascular and cerebrovascular events associated with long-term therapy with ibuprofen, it would be advisable to check the cardiovascular health status, especially in patients suffering from previous pathologies.
BRUFEN ® in tablets contains lactose, therefore it is not recommended in patients suffering from lactose intolerance, glucose-galactose malabsorption syndrome or lactase enzyme deficiency.
BRUFEN ® in cream contains excipients with allergenic and photosensitizing properties, therefore it is advisable to avoid direct exposure to sunlight.
PREGNANCY AND BREASTFEEDING
The use of BRUFEN ® during pregnancy and lactation is not recommended given the numerous studies in the literature that demonstrate the potential side and teratogenic effects of non-steroidal anti-inflammatory drugs on fetal and neonatal health.
Furthermore, it is worth considering that taking these drugs near the birth could increase the risk of bleeding in the mother, while reducing the frequency and intensity of uterine contractions.
Interactions
Although clinical practice has not shown clinically relevant drug interactions so far, the scientific literature defines a series of active ingredients whose concurrent use of ibuprofen could contribute to the occurrence of possible side effects.
More precisely the concomitant assumption of:
- ACE inhibitors and angiotensin II antagonists, could increase the risk of kidney failure;
- Analgesics, could alter the therapeutic profile of diclofenac;
- Antibiotics, given the cytochromial metabolism they face, could be associated with the appearance of side effects linked to the unpredictable increase in blood concentrations of the antibacterials used;
- Oral anticoagulants or antidepressants that inhibit serotonin reuptake, would be responsible for an increased risk of bleeding;
- Corticosteroids and other NSAIDs could increase the side effects expected for anti-inflammatory therapy, especially for the gastric mucosa;
- Methotrexate, would be potentially toxic, due to the increase in blood concentrations of this drug.
Contraindications BRUFEN ® Ibuprofen
Taking BRUFEN ® is contraindicated in patients who are hypersensitive to the active substance or to one of its excipients, hypersensitive to acetylsalicylic acid and other analgesics, suffering from asthma, nasal polyposis, liver failure, renal to cardiac, intestinal bleeding, ulcerative colitis or previous history for the same diseases.
Undesirable effects - Side effects
Despite taking BRUFEN ® according to the appropriate medical indications, it is generally well tolerated, the scientific literature and a careful post-marketing monitoring, have highlighted a series of possible adverse reactions linked to the use of ibuprofen.
These may be of interest:
- The gastrointestinal system with nausea, vomiting, abdominal pain, colitis, diarrhea, constipation and in severe cases gastritis and peptic ulcers;
- The hematologic apparatus with thrombocytopenia, neutropenia, haemolytic anemia and hematocrit reduction;
- The nervous system with vertigo, headache, irritability, drowsiness, depression, insomnia, mental confusion and difficulty concentrating;
- The skin with rash, erythema and rash;
- The cardiovascular system with edema, hypertension and heart failure in severe cases.
Note
BRUFEN ® can be sold exclusively with a medical prescription.