What is Effentora?
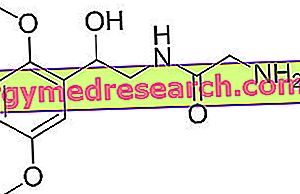
Effentora is a drug containing the active substance fentanyl, available as orosoluble tablets (which dissolve in the mouth), containing 100, 200, 400, 600 or 800 micrograms of fentanyl.
What is Effentora used for?
Effentora is indicated for the treatment of "breakthrough pain" (temporary pain exacerbations) in adults with cancer, already treated with opioid drugs (a group of painkillers that include morphine and fentanyl) for chronic pain due to cancer. Breakthrough pain occurs when the patient complains of sudden pain in addition to the basic pain. despite ongoing treatment with pain medication.
The medicine can only be obtained with a prescription.
How is Effentora used?
Effentora treatment should be started and maintained under the guidance of a doctor experienced in treating opioid therapy in cancer patients.
Effentora must be taken at the beginning of the painful episode. The tablets must be removed from the package and placed immediately in the mouth, above a molar, between the cheek and the gum. The tablet generally dissolves within 14-25 minutes, releasing the active ingredient, which is absorbed directly into the blood. After 30 minutes, any tablet residues can be swallowed with a glass of water. The tablets should not be broken or crushed; moreover they must not be sucked, chewed or swallowed whole. Patients should not take food or drink while holding the tablet in their mouth.
At the beginning of therapy with Effentora, the doctor must identify, on a case-by-case basis, the appropriate dose that can provide adequate relief of the patient's pain and, at the same time, reduce the side effects. As a rule, the initial dose is one 100 microgram tablet, which can be increased until the optimal maintenance dose is reached. During dose adjustment the patient should be monitored carefully.
The final dose should not include more than two tablets, but it can be reviewed if the patient has more than four episodes of breakthrough pain per day. Doses above 800 micrograms have not been evaluated in clinical studies. It is necessary to wait at least 4 hours before treating another episode of pain.
For more information, see the package leaflet.
How does Effentora work?
The active substance in Effentora, fentanyl, is an opioid (a powerful painkiller linked to morphine). It is a well known substance, used for many years to control pain. In Effentora, fentanyl is administered through an orosoluble tablet, so it is absorbed through the mucous membrane of the mouth. Once in the blood, fentanyl acts on receptors in the brain and spinal cord, preventing pain.
How has Effentora been studied?
Since fentanyl has been used for many years, the pharmaceutical company presented data taken from the scientific literature, as well as from its own studies.
The effects of Effentora in the treatment of breakthrough pain were analyzed in two main studies involving a total of 150 adult cancer patients receiving opioid therapy. The first study involved 72 patients, the second 78. In both studies, each patient was treated during 10 different painful episodes: in seven of these episodes Effentora was given, while in the remaining three episodes each patient received a placebo (dummy treatment). The main measure of effectiveness was the change in pain intensity over the first 30 or 60 minutes after taking the tablet. Each patient assigned a score based on an 11-point scale to the intensity of his or her pain.
What benefit has Effentora shown during the studies?
In both studies Effentora was more effective than placebo in reducing pain. In the first study, the pain intensity was reduced by an average of 3.2 points in the 30 minutes after taking Effentora and 2.0 points after taking the placebo. In the second study, there was a reduction in pain intensity of 9.7 points in the 60 minutes after taking Effentora and 4.9 points with placebo.
What is the risk associated with Effentora?
The most common side effects with Effentora (seen in more than 1 patient in 10) are sensation of skidding or instability, nausea and reactions at the site of application of the tablet including pain, ulcers, irritation, abnormal sensations, numbness, redness, swelling and blisters. Effentora can also cause side effects commonly seen with other opioids, which however tend to decrease or disappear with continued use of the medicine. The most serious adverse reactions consist in respiratory depression (slowing down or stopping breathing), circulatory depression (reduction in the frequency of heartbeats), hypotension (decrease in blood pressure) and shock (insufficient blood supply to the tissues). Patients should therefore be monitored to prevent such effects. For the full list of all side effects reported with Effentora, see the Package Leaflet.
Effentora should not be used in people who may be hypersensitive (allergic) to fentanyl or any of the other substances. It must also not be used in patients who are not already taking opioid painkillers as a maintenance therapy for pain control, in patients suffering from severe respiratory problems or from a serious illness that causes the obstruction of the lungs.
Effentora should be used with caution in patients with moderate or severe problems with their liver or kidneys.
Why has Effentora been approved?
The Committee for Medicinal Products for Human Use (CHMP) decided that Effentora's benefits are greater than its risks for the treatment of breakthrough pain in cancer adults, already on maintenance therapy with an opioid for chronic cancer pain. The Committee recommended that Effentora be given marketing authorization.
What measures are being taken to ensure the safe use of Effentora?
Effentora's manufacturing company will provide information material to ensure that healthcare professionals are aware of the potential abuse of the medicine. The company will also remind healthcare professionals how to use the medicine safely and will disclose the risks arising from accidental exposure to fentanyl.
More information on Effentora:
On 4 April 2008, the European Commission issued a marketing authorization for Effentora to Cephalon Europe, valid throughout the European Union.
The full EPAR for Effentora can be found here.
Last update of this summary: 04-2008.