Remaining on the subject of xenobiotic metabolism, we now go on to illustrate the metabolism of a compound used in agriculture as an insecticide fumigant, in chemical industries for the production of dyes and in pharmaceutical industries.
The compound taken under observation is DIBROMOETHANE . This compound is metabolized by conjugation with glutathione. Glutathionetransferase transports glutathione to the end of the alkyl chain that presents two bromine molecules at the beginning and end. At this point the ring closes also losing the second bromine molecule and an ion known as IONE EPISULPHONE is formed, which is very reactive with the bases of DNA and which for this - like all species reactive with DNA - favors the onset of neoplasms.
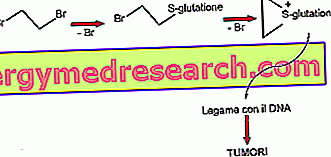
This particular interaction confirms a theory that was formulated several years ago, known as the electrophilic theory of chemical carcinogens . Most of these synthetic chemical compounds (hence taking into consideration both the original molecule and the metabolites) is highly electrophilic. To restore a balance of charge these electrophilic compounds must therefore react with nucleophilic groups, which can be found for example in DNA. Over the years, this theory has been further confirmed by numerous studies.



