Generality
Multiple myeloma is a malignant tumor that affects certain cells of the immune system, creating damage to various organs and tissues. It generally develops in people over the age of 50 and is often accompanied by pain, fractures and bone destruction.
Multiple myeloma is diagnosed with blood and bone marrow tests, protein electrophoresis in urine and radiological investigations. Disease remissions can be induced with steroids, chemotherapy, proteasome inhibitors, immunomodulatory drugs (such as thalidomide or lenalidomide) and stem cell transplantation.
Causes
The causes that cause multiple myeloma are in part unknown. From the medical statistics it was discovered that the incidence increases in subjects exposed to ionizing radiation or to particular chemical substances (petroleum derivatives and other hydrocarbons, solvents, pesticides, asbestos etc.). Family genetic factors, repeated antigenic stimulation and viral agents can also participate in the etiology of multiple myeloma.
Symptoms
To learn more: Multiple Myeloma Symptoms
Multiple myeloma occurs mainly in old age (over 50 years). Signs and symptoms can vary greatly from person to person, as many organs can be affected by the disease. At the onset, the condition may not cause any symptoms. As the disease progresses, symptoms related to plasma cell organ infiltration and excessive production of monoclonal immunoglobulins are likely to occur:
- Bone pains. Bone pain affects almost 70% of patients with multiple myeloma and is the most common symptom. It is mainly located in the spine, pelvis, ribs, long bones and skull. The initial symptomatology is "pseudoreumatic", while an acute and persistent localized pain could indicate a pathological bone fracture. Vertebrae involvement can lead to spinal cord compression. In multiple myeloma, bone damage is secondary to the appearance of "lytic" type lesions and osteopenia related to the proliferation of myelomatous plasma cells in the medullary cavity. The cells of multiple myeloma, in fact, produce various factors (gathered in the denomination OAF, Osteoclast Activating Factor s) that allow the osteoclasts to accelerate the demolition of the bone tissue, by reabsorption. Since osteoblasts ("bone producers") do not undergo the same proliferative stimulus, bone mass is progressively reduced. This alters the structure of the tissue, making the bones fragile and prone to fractures, osteoporosis and crushing (vertebrae). From the radiological point of view, the bone rearrangement is evident due to the appearance of "perforated" spots, which coincide with the areas in which osteocondensation is absent. Increased bone lesions can also increase the release of calcium in the blood (hypercalcemia).
- Hypercalcemia . A high level of calcium in the blood affects nerve function and causes excessive thirst, nausea, constipation, loss of appetite and mental confusion. Hypercalcemia is due to the infiltration of malignant cells into the bones.
- Kidney failure. Several factors intervene in conditioning kidney function. High levels of abnormal immunoglobulins, 'monclonal abnormalities (proteins? ‹M), can cause tubular lesions following their deposition in the glomeruli (Bence Jones proteinuria). The increased bone resorption leads to hypercalcemia and causes nephrocalcinosis, thus contributing to renal failure. Other causes include hyperuricemic nephropathy (from deposition of uric acid), local infiltration of tumor cells, recurrent infections (pyelonephritis) and amyloidosis.
- Anemia. In multiple myeloma, the deficiency of red blood cells results from the failure to produce "healthy" blood cells in favor of tumor clones in the bone marrow. Anemia can lead to asthenia, general weakness and difficulty in breathing.
These four problems, common to multiple myeloma, are often referred to by the acronym CRAB, which refers to levels of Calcium, Renal failure, Anemia and bone damage → C = Calcium (elevated), R = Renal failure, A = Anemia, B = Bone lesions
Other signs and symptoms of multiple myeloma may include:
- Infections : the reduction of white blood cells (leucopenia) leads to immune deficiency, therefore to a lower resistance to infections. These can be of different clinical severity (pneumonia, sinusitis, infections of skin, bladder or kidney) and represent the main cause of death of patients with multiple myeloma. The period of greatest risk for the onset of infections includes the initial months, after the start of chemotherapy.
- Alterations of haemostasis : the lack of platelets (thrombocytopenia) causes alterations in the coagulation process, which manifests itself with a marked tendency to bleed. Overall, the platelet half-life is reduced and the interaction with the vasal endothelium is altered. Other abnormalities of haemostasis result from reduced fibrinolysis.
- Hyperviscosity syndrome : in some cases of multiple myeloma, there is an increase in the value of plasmatic viscosity, which causes hemorrhagic symptoms, neurological disorders and coronary ischemia.
- Neurological disorders : the neurological manifestations associated with multiple myeloma are heterogeneous. The appearance of neuritic pains and weakness or numbness in the limbs are the most common symptoms. Finally, there may be radicular pain and spinal cord compression due to vertebral involvement, or carpal tunnel syndrome and other neuropathies caused by infiltration of amyloid deposits at the peripheral nerve level.
Diagnosis
The doctor can detect signs of multiple myeloma through blood and urine tests performed during a routine physical examination. If the patient is asymptomatic, these laboratory tests can be repeated regularly, in order to monitor the evolution of the disease and determine the best time to start treatment. Organ involvement is an important diagnostic criterion for multiple myeloma.
Laboratory tests
In many cases, the blood count establishes the presence of anemia (Hb <10g / dl). The peripheral blood smear shows stacked red blood cells (red cells), a consequence of the increased plasma viscosity. Often, neutropenia and thrombocytopenia coexist, which worsen in the terminal stages of the disease.
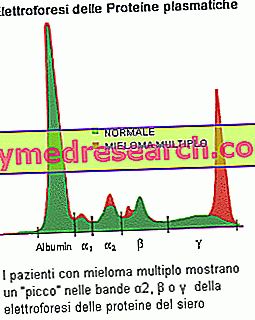
The electrophoresis of serum proteins is able to demonstrate the presence of paraproteins produced by the tumor clone: the suspicion of myeloma is founded if the band (peak) is relevant in the electrophoretic profile, with component M> 30 g / L. Another common finding is the proteinuria of Bence Jones, characterized by the presence in the urine of paraproteins consisting of light chains of monoclonal type. Quantitative measurements of the M component are necessary to establish a diagnosis of multiple myeloma and monitor the disease. Blood tests establish an increased rate of erythrocyte sedimentation (ESR) and lead to alterations in calcium levels (elevated in people with advanced multiple myeloma), albumin (low levels correlated with advanced myeloma), uric acid (uricemia) and creatinine (increase due to reduced kidney function). Your doctor may also perform other tests to check for the presence of beta-2-microglobulin, another protein produced by myeloma plasma cells. This can be a useful indicator in the staging of a patient: high levels indicate a more advanced disease and a worse prognosis.
Imaging techniques
The radiological survey shows the involvement of the skeleton. Magnetic resonance imaging is more sensitive than standard radiography in detecting lytic lesions and is able to define the presence of bone rearrangements early.
Bone marrow examination
Bone marrow examination shows numerous plasma cells: a diagnostic requirement for the definition of multiple myeloma is that the amount of tumor clones is greater than 10%. A portion of the sample is also tested to evaluate the presence of characteristic karyotype alterations, by conventional cytogenetics or the use of fluorescent in situ hybridization techniques (FISH). The chromosomal anomalies most frequently found in multiple myeloma involve chromosomes 1, 3, 5, 11, 13 and 14. In particular, the monosomy of chromosome 13 correlates with a reduced survival, associated with a high rate of proliferation and drug resistance.
Staging and prognosis
Diagnostic investigations can confirm the clinical picture of multiple myeloma. Furthermore, the results of these tests allow the doctor to classify the disease in stage 1, 2 or 3. People with stage 3 myeloma have one or more signs of advanced disease, including more tumor clones and kidney failure. Furthermore, the outcome of bone marrow biopsy allows the doctor to determine the patient's overall risk profile and develop the best treatment plan.
| Staging of multiple myeloma (according to Durie-Salmon) | ||
| Clinical stage | Parameters | Tumor mass (number of cells) |
| Stage 1 | All the following:
| <0.5x1012 / m2 |
| Stage 2 | None of the criteria of stage I and III | 0, 5-1, 2x1012 / m2 |
| Stage 3 | One or more of the following:
| > 1, 2x1012 / m2 |
The stages 1, 2 and 3 of the Durie-Salmon staging system can be further subdivided into A or B, depending on the serum creatinine:
- A: creatininemia <2 mg / dl (<177 mmol / L)
- B: serum creatinine> 2 mg / dl (> 177 micromol / L)
Recently, another staging system has been proposed called the International Multiple Myeloma Staging System .
International prognostic index for myeloma (published by the International Staging System - 2005) | ||
Stadium | criteria | Average survival (months) |
THE |
| 62 |
II | h42-microglobulin <3.5 mg / L and albumin <3.5 g / dl or β2-microglobulin 3.5-5.5 mg / L independently of serum albumin | 44 |
III | β2-microglobulin ≥ 5.5 mg / L | 29 |
Treatment
To learn more: Medicines for the treatment of Multiple Myeloma
The treatment of multiple myeloma is focused on therapies that reduce the clonal population. If the disease is completely asymptomatic, management is limited to clinical observation. In the presence of symptoms, treatment can help relieve pain, control complications, stabilize the condition and slow down the progression of the tumor.
First-line therapy
In recent years, high-dose chemotherapy with autologous hematopoietic stem cell transplantation (autograft) has become the most appropriate therapeutic option for patients under the age of 65. Before the transplant, an initial cycle of induction chemotherapy is administered, able to reduce the neoplastic mass. The most commonly used regimens include the administration of thalidomide (+ dexamethasone), bortezomib and lenalidomide (+ dexamethasone). Treatment with high doses of chemotherapy and stem cell support (autotransplantation) is not curative, but it can prolong overall survival and determine complete remission. Allogeneic stem cell transplantation, ie from a healthy person to the affected patient, represents a potential cure, but is only available for a small percentage of clinical cases. Patients over 65 years of age and with significant concomitant disease often cannot tolerate stem cell transplantation. For these people, the standard treatment often involves chemotherapy with melphalan (for 4-7 days) associated with prednisone (5-10 days), in cycles repeated every 6 weeks, followed by a period of rest. In some patients, the association of this protocol with new therapeutic regimens, which include, for example, bortezomib administration may be useful. This drug is a proteasome inhibitor (a multi-protein complex present in all cells), which allows for complete clinical responses in patients with refractory or rapidly progressing disease. The mechanism of action of bortezomib is based on the induction of apoptosis in tumor cells, blocking the action of proteosomes. A significant clinical response is found in approximately 50% of cases
Supportive therapy
In addition to the direct treatment of multiple myeloma, bisphosphonates (eg pamidronate or zoledronic acid) are regularly administered to control lytic lesions and prevent bone fractures. Red cell-based transfusions and erythropoietin are useful in correcting the symptoms associated with anemia, while the administration of platelet concentrates can prevent bleeding caused by thrombocytopenia. Steroids and bisphosphonates also become important in the treatment of hypercalcemic crises, while antibiotics are supportive in the management of infections.



