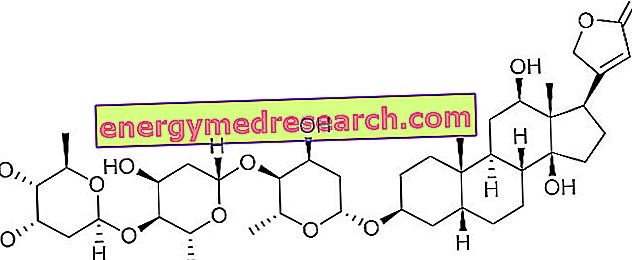
PLACENTEX ® is a Polidesoxyribonucleotide drug
THERAPEUTIC GROUP: Cicatrizzanti
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications PLACENTEX ® Polidesoxyribonucleotide
PLACENTEX ® is used in the treatment of skin and connective lesions associated with dystrophic and dystrophic-ulcerative pathologies.
Mechanism of action PLACENTEX ® Polydesoxyribonucleotide
PLACENTEX ® is a drug characterized by the complex mechanism of action, which sees its active ingredient Polideoxiribonucleotide at the center of numerous biological activities.
More precisely, once extracted through patented laboratory procedures and applied topically, the Polideoxiribonucleotide, consisting of several deoxyribonucleotides joined together by phosphodiester bonds, reaches the phlogistic site with high tropism, interacting with elements such as platelets and fibronectin, defining the formation of molecular complexes able to facilitate cell regeneration.
However, the inducing action on the cell cycle seems to be associated with the ability of the active principle to activate alternative signal pathways, capable of supporting gene expression, optimizing DNA synthesis and the subsequent process of cell proliferation and tissue regeneration.
Studies carried out and clinical efficacy
POLIDEOSSIRIBONUCLEOTIDE AND ULCERS IN THE DIABETIC PATIENT
Cardiovasc Hematol Agents Med Chem. 2009 Oct; 7 (4): 313-21.
Very interesting clinical work that demonstrates how Polideoxiribonucleotide can optimize angiogenesis in the cutaneous regions affected by diabetic ulcers, definitely improving the clinical picture.
POLIDEOSSIRIBONUCLEOTIDE IN THE TREATMENT OF VARICOCELE
Fertil Steril. 2012 Jan; 97 (1): 165-8. doi: 10.1016 / j.fertnstert.2011.10.007. Epub 2011 Nov 17.
Arena S, Minutoli L, Arena F, Nicotine PA, Romeo C, Squadrito F, Altavilla D, Morgia G, Magno C.
Experimental study that tests the use of Polideoxiribonucletoide in the treatment of varicocele, observing the ability of the latter to induce neoangiogenesis by improving testicular function.
POLIDEOSSIRIBONUCLEOTIDE FROM HUMAN PLACENTA
J Pharm Biomed Anal. 1996 Aug; 14 (11): 1555-60.
A dated study that evaluates the possible sources of Polideossiribonucletoride extractions, including human placenta, able to provide good quantities of this active ingredient with excellent tolerability.
Method of use and dosage
PLACENTEX ®
Topical ointment with 80 mg of Polideoxiribonucleotide per 100 g of product.
It is generally recommended to apply the correct amount of ointment once or twice a day to the region directly affected by the dystrophic or ulcerative process.
Warnings PLACENTEX ® Polidesoxyribonucleotide
Therapy with PLACENTEX ® should be preceded by a careful medical examination to ascertain the patient's general health condition and the nature of the described skin lesions.
The topical use of PLACENTEX ®, especially if it lasts a long time or carried out on large cutaneous regions, could determine the onset of local adverse reactions, including those of an allergic nature.
It is recommended to store the drug in a cool, dry place and out of reach of children.
PREGNANCY AND BREASTFEEDING
The use of PLACENTEX ® during pregnancy and breastfeeding should take place under strict medical supervision and in cases of real need.
Interactions
It is recommended to avoid contextual use of other drugs topically
Contraindications PLACENTEX ® Polidesoxiribonucleotide
The use of PLACENTEX ® is contraindicated in patients who are hypersensitive to the active substance or to one of its excipients.
Undesirable effects - Side effects
PLACENTEX ® is generally safe and very well tolerated.
The local and clinically non-relevant adverse reactions associated with the use of this medicine are very rare.
Note
PLACENTEX ® is a non-prescription drug.