Morphology
At a macroscopic level, a variable degree of cortical atrophy (reduction of tissue or organ mass) can be observed in Alzheimer's disease, characterized by enlargement of the parietal furrows, more accentuated in the frontal, temporal and parietal lobes. This atrophy is compensated by an enlargement of the ventricular cavities secondary to the loss of parenchyma (figure 1). In particular, in the advanced stages of the disease, the structures of the medial temporal lobe, including the hippocampus, the entorhinal cortex and the amygdala, atrophy severely, given their involvement starting from the early stages of the pathology.
Furthermore, Alzheimer's disease also presents microscopic alterations, known as extracellular senile plaques and intracellular neurofibrillary clusters, which represent the basis of histological diagnosis. With the progression of the disease, then, there is a serious neuronal loss accompanied by gliosis (circumscribed or diffuse proliferation, reactive in nature, of neuroglia cells, that is, cells that constitute the supporting stroma of nervous tissue) in the same regions where the presence of neurofibrillary plaques and clusters is greater.
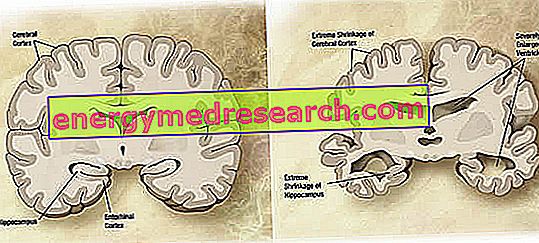
Figure 1. Coronal section of brain: differences between normal brain and brain affected by AD (wikipedia image source).
Pathogenesis
Alzheimer's disease is characterized mainly by two typical lesions: the extracellular accumulation of senile plaques consisting mainly of the β-amyloid peptide (Aβ) and intraneuronal neurofibrillary clusters, formed by hyperphosphorylated tau protein.
- Senile plaques can be found at the level of brain areas such as hippocampus, amygdala and neocortex.
Aβ peptide derives from a proteolytic cut of the amyloid protein precursor (APP), by β-secretase. This cut generates a terminal carboxy fragment of 99 residues (CTF or C99) which is subsequently cut by the β-secretase to generate Aβ fragments, having different lengths. It is known that the most abundant species of Aβ is the Aβ40 fragment. Another known fragment formed from the proteolytic cut, less abundant than Aβ40, is Aβ42, more prone to form amyloid fibrils, which accumulate as an Aβ species in the brain of an individual with Alzheimer's disease.
- Another component present in Alzheimer's disease is represented by neurofibrillary clusters consisting of bundles of filaments present in the cytoplasm of neurons. Neurofibrillary clusters are insoluble and seem to be resistant to proteolysis processes in vivo, thus remaining present in tissue sections even for a long time after neuronal death. Observing the structure, the fibrillar clusters are made up of double helix filaments and linear filaments of similar composition. Analyzing the composition, the double helix strands are mostly made of hyperphosphorylated tau protein. Tau is an axonal protein associated with microtubules which facilitates its assembly.
Other important neuropathological changes present in Alzheimer's disease include mitochondrial dysfunction, oxidative neuronal damage, synaptic loss and axonal degeneration.
Neurochemical aspects
As already explained, Aβ peptide, deriving from the proteolytic cut of the APP precursor, represents a neurotoxic component of Alzheimer's disease . Specifically, it has been hypothesized that Aβ may be important for normal brain functions and, if it overcomes certain concentrations, it may become neurotoxic. In addition, both the aggregates and the different isoforms of Aβ could have a different biological, physiological or pathological role, determining and participating in the subsequent stages of the disease. It has been observed that Aβ acts as a neuromodulator, influencing the release of some neurotransmitters in the absence of obvious signs of neurotoxicity.
For example, the neuromodulatory role of Aβ, in a physiological context, could have an important meaning for the correct balance of the neurotransmitter system. It is well known that this system is made up of neurotransmitters, substances that convey information between the cells that make up the nervous system, the neurons, through synaptic transmission.
In pathological conditions, on the other hand, Aβ-mediated synaptic transmission could be related to alteration of neurotransmission before neurodegenerative events. As a result of these changes, early cognitive and non-cognitive disorders may arise, based on the neurotransmitter systems affected and the different brain areas involved.
The alterations of the neurotransmitter systems and the signal transduction mechanism in the brains of individuals suffering from Alzheimer's disease are very complex. One of the systems that appears to be altered concerns the cholinergic signaling system, which involves the neurotransmitter acetylcholine. Indeed, it has been shown that individuals suffering from Alzheimer's disease show reduced cholinergic transmission at the level of the cortex and hippocampus, important brain areas dedicated to phenomena such as learning and memory. In addition to this neurotransmitter system, alterations in the noradrenergic, serotonergic, as well as glutamate and GABA systems have been observed in Alzheimer's disease.