Reverse osmosis is a simple and economical process to purify water from impurities of various kinds. This technique exploits the potential of a semipermeable membrane, for example a cellulosic film, which can be passed through by the solvent (in our case the water) but not by solutes (impurities). In natural conditions, if two containers are separated by this membrane, solvent is transferred from the area in which it is more concentrated to that in which it is present in lower concentrations. To take us back to the practical example, water passes from the container where it is purer (eg distilled water) to that in which it has a lower degree of purity (eg saline water). This step stops when the two containers reach the same ratio between water and impurities.
In reverse osmosis, the container where the water is less pure is applied such pressure that it overcomes its natural tendency to enter this compartment. In this way there is an inversion of the natural osmotic flow and, returning to the previous example, the passage of water in the sense "concentrated solution (saline water) → diluted solution (distilled water)".
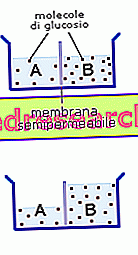
To better clarify the concept of osmosis, let us imagine having a vessel divided into two compartments of equal volume (A and B) by a semipermeable membrane (that is, permeable only to the solvent - in this case the water - and not to the solute, in this glucose case). In compartment A there is an aqueous solution in which a tablespoon of glucose was dissolved, while in part B we have an aqueous solution of equal volume in which three tablespoons of glucose were dissolved. This difference creates a concentration gradient for glucose on the sides of the membrane and, since this sugar cannot pass through it, the balance is reached with the passage of water from compartment A (where glucose is most diluted) to the compartment B (where it is most abundant). If you prefer, it can also be said that water passes through osmosis from the solution in which it is more concentrated (A) to that in which it is less concentrated (B).
THE

If a pressure higher than the osmotic pressure is applied in B, it is called reverse osmosis.

In addition to reverse osmosis the purifiers also exploit other types of filters, such as those with activated carbon (useful for eliminating chlorine) and UVA rays (which have a sterilizing action).
Purifiers that use the reverse osmosis process are widely used both in the domestic and industrial sectors, for example in the industrial desalination plants of seawater or in car washes (demineralized water does not leave stains on the bodywork).
The simplicity of this technique and the advantage of not requiring the addition of chemical substances, has made reverse osmosis the most widespread food water purification system, capable of removing almost all organic substances (including pathogenic and non-pathogenic microorganisms). ), but also a good part of the mineral salts. The water coming out of the reverse osmosis purifiers can therefore be considered oligomineral water, that is a water with low fixed residue (poor in mineral salts). The detractors of this method point their finger precisely against this characteristic, which would impoverish the water of precious minerals to the point of making it distilled and nutritionally "empty". In reality, very often, behind these criticisms lies the commercial need to supply purifiers that use alternative filtration methods. Let us not forget, in fact, that the contribution of water to the coverage of the daily needs of individual minerals is particularly modest, especially for the much-advertised oligomineral and minimally mineralized waters (if advertising highlights the benefits of these waters all day long, in reality highly questionable, because then they come to tell us that the water obtained by reverse osmosis "hurts" because it is too poor in minerals ?! For the same reasons, however, it is wrong to take advantage of excess mineral salts in tap water in order to favor the purchase of reverse osmosis purifiers.
The most commonly used minerals in this "trade war" are sodium and calcium. First of all, it must be said that drinking water can represent an important source of calcium even if, due to the variability of the content, it is difficult to estimate their contribution to the daily intake of calcium in individuals. The mineral water that the writer has in front of contains 34 mg / L of calcium, so it would be necessary to drink more than 30 liters to cover the calcium needs of an adult (drinking two liters a day his contribution to the coverage of the calcium needs is around 6%). With regard to the sodium content and its relationship with heaviness in the legs, swelling and cellulite, we expressed ourselves in a specific article; briefly, know that you will not solve these problems by using a reverse osmosis purifier. More generally, in the presence of an underlying pathology, the choice of mineral water should be entrusted to the doctor; there are, for example, mineral waters with a calcium content higher than 150 mg / L, which can cover up to one third of the daily requirement of this mineral (a characteristic that could make them useful in the presence of osteoporosis).