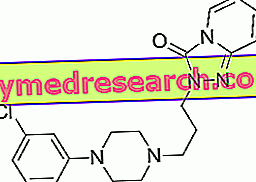
FAMVIR ® is a Famciclovir based drug
THERAPEUTIC GROUP: Antivirals for systemic use
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications FAMVIR ® Famciclovir
FAMVIR ® is indicated in the treatment of infectious diseases supported by viruses such as Herpes Simplex type 1 and 2 and Varicella Zooster Virus.
Mechanism of action FAMVIR ® Famciclovir
FAMVIR ® is a medicinal specialty based on Famciclovir, a prodrug of the most active Penciclovir, a nucleoside analogue of guanine and therefore active against high replication power viruses such as HSV and VZV.
Taken by mouth, Famciclovir is rapidly converted into Penciclovir, ensuring maximum plasma concentrations are reached within minutes of oral intake, and thus, following a homogeneous distribution in the various parts of the body, the carrying out of its therapeutic activity.
Upon reaching the host cell, Penciclovir undergoes phosphorylation processes operated primarily by viral enzymes and subsequently by cellular enzymes, which transform it into Penciclovir triphosphate, a nucleoside analogue of guanine that intercalates in the DNA chain and blocks the activity of viral DNA polymerase, preventing the progress of various proliferative processes.
All this translates into a prompt regression of the present symptomatology.
Studies carried out and clinical efficacy
FAMCICLOVIR AS AN ANTIVIRAL IN PATIENTS WITH ACUTE RENAL FAILURE
Int J Clin Pharm. 2013 Jan 1.
Case report demonstrating that Famciclovir may be safer and more effective than aciclovir as an antiviral in patients suffering from acute renal failure, greatly limiting potential kidney damage.
FAMCICLOVIR IN THE TREATMENT OF INFECTIONS BY HERPES ZOOSTER
J Dermatol. 2012 Nov; 39 (11): 902-8.
Work that demonstrates how Famciclovir can be effective and therefore recommended in immunocompetent patients with Herpes Zooster with medium-sized symptoms, significantly reducing the ongoing symptoms.
FAMCICLOVIR IN PREGNANCY
JAMA. 2010 Aug 25; 304 (8): 859-66. doi: 10.1001 / jama.2010.1206.
Interesting work that tests the potential risks of taking Aciclovir, Valaciclovir and Famciclovir for fetal health in the first trimester of pregnancy. These early data seem encouraging not showing a significant increase in birth defects.
Method of use and dosage
FAMVIR ®
125 mg - 250 mg and 500 mg Famciclovir coated tablets.
Both the dosage and the relative dosing schedule should be defined by a doctor experienced in the treatment of infectious diseases, considering a series of characteristics of the patient such as age, the possible presence of liver and kidney diseases, the overall health status, the severity of the clinical picture and clearly the therapeutic goals.
Warnings FAMVIR ® Famciclovir
The therapy with FAMVIR must necessarily be preceded by a careful medical examination designed to assess the prescriptive appropriateness of the drug, the possible presence of contraindications to the use of the Famciclovir or of conditions such as to require an adjustment of the doses.
Precisely for this reason it would be appropriate to pay particular caution to elderly patients or those suffering from liver and kidney diseases, for whom the incidence of adverse reactions is decidedly greater.
Note the high ability of contagion and inter-human transmission it would be appropriate to combine pharmacological therapy also the respect of a series of hygienic rules useful to limit the spread of the virus and the consequent use of antiviral drugs, whose abuse could facilitate the onset of mechanisms of resistance to drug therapy.
FAMVIR ® contains lactose, therefore its use is not recommended in patients with lactase enzyme deficiency, glucose-galactose malabsorption syndrome and lactose intolerance.
PREGNANCY AND BREASTFEEDING
The use of FAMVIR ® during pregnancy and in the subsequent period of breastfeeding is generally contraindicated, note the ability of Penciclovir to cross the blood-placental barrier and permeate the mammary filter, thus exposing itself in relevant concentrations to the fetus and to the infant.
For this reason, the possible use of FAMVIR ® in the above periods should be established by your gynecologist based on the cost / benefit ratio of the therapy itself.
Interactions
Although active ingredients capable of interacting with Penciclovir are not known at present, leading to clinically relevant adverse reactions, it would be advisable in any case to avoid the simultaneous intake of potentially hepatic and nephrotoxic drugs that could alter the pharmacokinetic characteristics of Famciclovir, increasing the risk of side effects serious.
Contraindications FAMVIR ® Famciclovir
The use of FAMVIR ® is contraindicated in patients hypersensitive to Aciclovir or to structurally related active ingredients rather than to excipients present in the drug.
Undesirable effects - Side effects
The use of FAMVIR ® could determine, with a certain frequency, the appearance of nausea, vomiting, diarrhea, headache, abnormal liver function and hypersensitivity reactions such as rash and itching.
Fortunately, clinically relevant adverse reactions are rare.
Note
FAMVIR ® is a prescription-only drug.