ORUDIS ® is a ketoprofen based drug
THERAPEUTIC GROUP: Non-steroidal anti-inflammatory and antirheumatic drugs
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications ORUDIS ® Ketoprofen
ORUDIS ® is indicated for the treatment of acute pain symptoms during osteo-articular and musculoskeletal inflammation.
Mechanism of action ORUDIS ® Ketoprofen
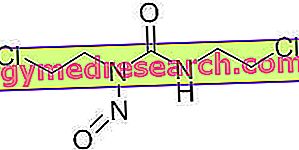
Ketoprofen, the active ingredient of ORUDIS ®, is a non-steroidal anti-inflammatory drug chemically derived from arylpropionic acid, commonly used in the treatment of musculoskeletal inflammatory diseases given the particular tropism of this molecule against synovies.
The therapeutic action of ketoprofen is guaranteed by two main mechanisms:
- The anti-inflammatory one, well characterized molecularly and attributable essentially to the inhibitory activity against cyclooxygenases, enzymes expressed during traumas of various nature and capable of mediating the transformation of membrane phospholipids such as arachidonic acid into chemical mediators with marked activity. inflammatory such as prostaglandins;
- The analgesic one, supported by the ability of ketoprofen to cross the blood-brain barrier, reaching the central nervous system and exerting a non-opioid pain-relieving action, probably linked to a variation in receptor sensitivity to molecules with algogenic activity.
Both dynamics of action support the pain-relieving and anti-inflammatory activity of ketoprofen, also facilitated by the excellent pharmacokinetic properties that allow the active ingredient taken orally to be effectively absorbed at the intestinal level, reaching the plasma peak within 90 minutes of intake, to distribute itself bound to plasma proteins, mainly at the level of synovial fluid and intra-articular, capsular and tendon tissues.
Renal elimination, in the form of inactive catabolites, is carried out following a classic process of hepatic glucuronation.
Studies carried out and clinical efficacy
1.THE KETOPROPHENES IN THE RHEUMATIC AND TRAUMATIC PAIN
J Pak Med Assoc. 1998 Dec; 48 (12): 373-6.
Very interesting work that compares the efficacy and safety of ketoprofen to that of diclofenac, observing how the use of ketoprofen intramuscularly can be more effective in the treatment of acute pathologies of rheumatic and traumatic origin, with more contained and clinically less collateral effects relevant.
2. KETOPROPHENES AND HEADACHE
Headache. 1997 Jan; 37 (1): 12-4.
Work demonstrating the efficacy of intramuscular use of 100 mg ketoprofen in the reduction of acute pain present in pathological conditions such as headache
3. KETOPROPHENES AS SYMPTOMATIC THERAPY IN RHEUMATOID ARTHRITIS
Clin Ther. 1994 Mar-Apr; 16 (2): 222-35.
Study that demonstrates how the use of prolonged release ketoprofen can be effective as symptomatic therapy during severe rheumatic diseases such as rheumatoid arthritis. However, in order to control the clinical course of the disease, it is necessary to associate an anti-inflammatory drug with an immunomodulatory or cytostatic activity.
Method of use and dosage
ORUDIS ®
Suppositories for 100 mg rectal use of ketoprofen;
Extended release capsules of 200 mg ketoprofen;
50 mg capsules of ketoprofen;
Topical gel with 5% ketoprofen;
Vials for intramuscular use of 100 mg of ketoprofen / 2 ml of solution.
The dosing schedule envisaged for the treatment of painful states on an inflammatory basis with ketoprofen varies significantly depending on the pharmaceutical format used, the patient's health conditions and the severity of the present clinical picture.
Consequently, the doctor should draft a specific therapeutic protocol for each case, optimizing the doses used according to the individual case.
In any case, it is advisable to use the minimum dose able to guarantee a remission of symptoms, in order to reduce the incidence of side effects.
Warnings ORUDIS ® Ketoprofen
It is useful to remember that the use of non-steroidal anti-inflammatory drugs such as ORUDIS ® must be understood as short-term symptomatological therapy, designed to overcome acute pain arising on an inflammatory basis.
The duration of therapy and the magnitude of the dose used in the treatment, significantly influence the incidence of side effects, thus suggesting the need to resort to minimal effective doses and short periods of therapy.
The intake of NSAIDs, especially in patients with liver, kidney, gastrointestinal and cardiovascular diseases, should be supervised by their doctor, who should periodically evaluate the state of functionality of the aforementioned organs and apparatuses, in order to limit the incidence of serious side effects.
It is useful to remember that the intake of ketoprofen by injection parenteral may be more frequently associated with the appearance of side effects from hypersensitivity, given the presence in ORUDIS ® of excipients with allergenic power.
ORUDIS ® in hard capsules contains lactose, therefore its intake is contraindicated in patients with lactose intolerance, lactase enzyme deficiency or glucose-galactose malabsorption syndrome.
ORUDIS ® in prolonged-release hard capsules contains sucrose instead, therefore special caution is recommended in patients suffering from metabolic diseases such as diabetes or glucose-galactose mal-absorption syndromes.
PREGNANCY AND BREASTFEEDING
The intake of ketoprofen, as well as that of other non-steroidal anti-inflammatory drugs, is not recommended during pregnancy due to the adverse reactions of the fetus and the mother.
In fact, different studies show that the induced absence of prostaglandins can compromise the normal embryonic and fetal development, increasing the incidence of malformations of the cardiovascular and pulmonary apparatus and of unwanted abortions.
The administration of NSAIDs in the terminal period of gestation could also complicate delivery, reducing the intensity and frequency of uterine contractions and simultaneously increasing the risk of bleeding in the parturient.
Taking ORUDIS ® is also contraindicated in the subsequent breastfeeding phase, given the possible secretion of ketoprofen in breast milk.
Interactions
Pharmacokinetic studies demonstrate how the pharmacokinetic and pharmacodimanic properties of ketoprofen can be altered by the simultaneous intake of other active ingredients, varying both the therapeutic efficacy of the drug and its safety profile.
The interactions to be paid particular attention to are those with:
- Oral anticoagulants and inhibitors of serotonin reuptake, due to the increased risk of bleeding;
- Diuretics, ACE inhibitors, angiotensin II antagonists, methotrexate and cyclosporine, due to potential nephrotoxic effects;
- Non-steroidal anti-inflammatory and corticosteroids, able to significantly increase lesions affecting the gastro-intestinal tract;
- Antibiotics, given the significant variations in terms of therapeutic efficacy and metabolism;
- Sulfonylureas, given the possible hypoglycemic action.
Contraindications ORUDIS ® Ketoprofen
Taking ORUDIS ® is contraindicated in patients who are hypersensitive to the active substance or to one of its excipients, hypersensitive to acetylsalicylic acid and other analgesics, suffering from hepatic, renal and cardiac insufficiency, bleeding diathesis, intestinal bleeding, ulcerative colitis, Crohn's or past history for the same conditions.
Undesirable effects - Side effects
The use of non-steroidal anti-inflammatory drugs such as ketoprofen is often associated with the onset of numerous side effects distributed among the various organs and systems, with greater frequency in patients predisposed or suffering from renal, liver and gastrointestinal pathologies.
Among the most affected apparatuses we find that:
- Gastrointestinal with nausea, vomiting, diarrhea, constipation, gastritis and in more serious cases ulcers with possible perforation and hemorrhages;
- Central with the onset of headache, dizziness and drowsiness;
- Dermal subject to hypersensitivity manifestations such as rash, urticaria, angioedema, bullous reactions and photosensitivity.
Of considerable importance are also the results from various studies, which show that prolonged use of NSAIDs can increase the incidence of renal, liver and cardiovascular diseases, or aggravate the clinical course in patients with a current disease.
Note
ORUDIS ® is salable only with a medical prescription.