What affects arteriolar resistance and glomerular filtration rate?
The control of the resistance opposed by the afferent and efferent arterioles to the renal glomerulus depends on numerous factors, both systemic and local.
The self-regulation of glomerular filtration speed involves several intrinsic nephron mechanisms, among which we recall:
- myogenic response: intrinsic ability of vascular smooth muscle to respond to pressure changes.
If stretched by the systemic pressure increase, the smooth muscle of the afferent arterioles responds by contracting; in this way it increases the resistance to the flow by reducing the quantity of blood passing through the arteriole and with it the hydrostatic pressure exerted on the walls of the glomerular capillaries → the filtration rate is reduced.
Conversely, if the systemic arterial pressure decreases, the arteriolar muscle relaxes and the vessel expands in a maximal manner; thus increases the flow of blood within the glomerulus and with it also increases the glomerular hydrostatic pressure and the filtration rate. It should be pointed out, however, that vasodilation is not as effective as vasoconstriction, since under normal conditions the afferent arteriole is already rather dilated. It allows the myogenic response to be able to compensate well for the increase in systemic arterial pressure, and a little less for a possible drop.
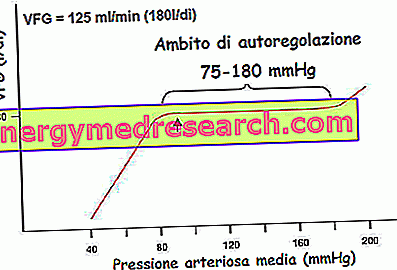
- tubulo-glomerular feedback: the flow variations of the liquid that crosses the final stretch of the loop of Henle and the initial tract of the distal tubule influence the glomerular velocity. In the loop of Henle processes of reabsorption of water and sodium and chloride ions take place. The particular folded structure of the nephron causes the final portion of the loop of Henle to pass between the afferent and efferent arterioles to the renal glomerulus. In this region between the two structures a structural and functional relationship is established such as to constitute a real "apparatus" defined "juxtaglomerulare apparatus. In the points where they come into contact, both the arteriolar walls and those of the tubule have a modified structure that In particular, the modified tubular structure consists of a cell plate called the dense macula, while the adjacent wall of the afferent arteriola contains specialized smooth muscle cells, called granular cells (or juxtaglomerular or JG). they secrete renin, a proteolytic hormone involved in the hydrosaline balance, with hypertensive effects.
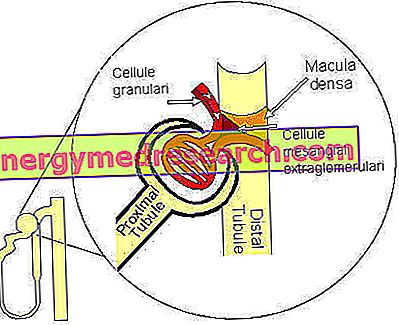
The control of the resistance of afferent and efferent arterioles is also regulated by systemic factors. On the other hand, one of the main functions of the kidney is to regulate systemic arterial pressure, so it is important that the glomeruli receive any changes in systemic arterial pressure and adapt the VFG accordingly. These changes are transmitted to the kidney endocrine and nervous.
The nervous control of VFG is entrusted to sympathetic neurons that innervate both the afferent and the efferent arterioles. Sympathetic stimulation, mediated by the release of adrenaline, causes vasoconstriction, especially of the afferent arteriola. Consequently, a strong activation of the sympathetic, consequent for example to a strong hemorrhage or to a severe dehydration, determines a contraction of the afferent and efferent arterioles to the glomeruli, reducing both the speed of glomerular filtration and the blood flow to the kidneys. In this way we try to keep the water volume as high as possible.
The endocrine control of VFG is entrusted to different hormones. In addition to circulating adrenaline, whose vasoconstrictor effects have just been described, arteriolar resistance is also increased by angiotensin II. However, in the latter case, vasoconstriction mainly affects the efferent arterioles, so that the increase in pressure in the glomerular capillaries increases the glomerular filtration rate. Among the vasodilatory substances that oppose the vasoconstrictor effect of the sympathetic nervous system and angiotensin II, we recall some prostaglandins (PGE2, PGI2, Bradykinin), which reduce the resistance to the flow offered, especially of afferent arterioles. This results in an increase in the glomerular filtration rate. Nitric oxide also exerts a vasodilating action at the arteriolar level.
The endocrine action takes place at the level of the podocytes or mesangial cells. The contraction or relaxation of the latter, which we remember being placed in the spaces surrounding the capillaries of the renal glomeruli, modifies the area of the capillary surface available for filtration. The podocytes, on the other hand, change the size of the glomerular filtration cracks; if these widen, the filtration surface increases, therefore the glomerular filtration rate is also raised.
Watch the video
X Watch the video on youtube