FALVIN ® is a drug based on Fenticonazole
THERAPEUTIC GROUP: Antimycotics for topical use
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications FALVIN ® Fenticonazole
FALVIN ® is indicated in the treatment of dermatomycoses sustained by dermatophytes sensitive to imidazole derivatives, even when complicated by bacterial superinfections with Gram positive bacteria, and in the treatment of candidiasis of genital mucous membranes.
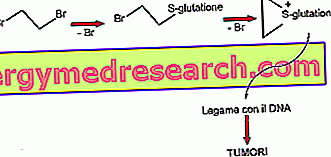
Mechanism of action FALVIN ® Fenticonazole
Fenticonazole, active ingredient of FALVIN ®, is an imidazole derivative therefore characterized by a sensitive fungicidal, fungistatic and bactericidal activity against Gram positive organisms.
Applied topically, Fenticonazole easily penetrates the cellular structures of bacteria and fungi, inhibiting the activity of enzymes such as the 14 alpha methyl lanosterol demethylase, involved in the synthesis of key molecules for cellular activity such as Ergosterol, a prominent element of dermatophyte plasma membrane.
In addition to the impairment of the normal cellular network due to the disorganization of the plsamatic membrane, the clinical efficacy of Fenticonazole is due to its fungicidal and bactericidal capacity linked to the accumulation of toxic catabolites derived from the inactivity of the aforementioned enzymes.
The very low systemic absorption of the active principle makes FALVIN ® therapy generally safe and well tolerated.
Studies carried out and clinical efficacy
CLINICAL EFFECTIVENESS AND SAFETY OF FENTICONAZOLE IN THE TREATMENT OF VAGINAL CANDIDIASIS
Srp Arh Celok Lek. 2012 Jul-Aug; 140 (7-8): 469-74.
Study conducted on over 400 women suffering from gynecological pathologies supported by various Candida species, which reaffirms the clinical efficacy of Fenticonazole in the treatment of the aforementioned pathological conditions and good tolerability.
FLUCONAZOLO VS FENTICONAZOLO
Minerva Ginecol. 2012 Apr; 64 (2): 89-94.
Study demonstrating the greater clinical efficacy of fenticonazole in the treatment of vaginal candidiasis compared to other imidazole derivatives such as fluconazole, also given the intense antipruritic activity
RAPIDITY OF ACTION OF THE FENTICONAZOLE
Comparison of single administration with an ovule of 600 mg fenticonazole versus 500 mg clotrimazole vaginal pessary in the treatment of vaginal candidiasis.
Wiest W, Azzollini E, Ruffmann R.
.
Italian work that demonstrates not only the clinical efficacy of Fenticonazole in the treatment of vaginitis but also its rapid action, important in the timely improvement of the health conditions of affected patients.
Method of use and dosage
FALVIN ®
Cream for cutaneous use at 2% of Fenticonazole nitrate;
Solution with nebulizer of 2 g of Fenticonazole per 100 ml of solution;
Vaginal cream with 2% Fenticonazole nitrate;
Ovules for vaginal use of 200 mg of Fenticonazole nitrate;
Vaginal lavender 200 mg of Fenticonazole nitrate per 100 ml of product.
The dosing schedule and the choice of pharmaceutical format should be defined by the doctor based on the patient's clinical conditions and the therapeutic goals to be achieved.
Usually the application of the appropriate quantity of product directly on the region to be treated for two times a day is sufficient to guarantee a prompt remission of the symptomatology.
Cream: indicated for the treatment of hairless skin and skin folds;
Solution with nebulizer: indicated for the treatment of scalp manifestations;
Preparations for vaginal use: indicated in the treatment of candidiasis of the genital mucous membranes.
Warnings FALVIN ® Fenticonazole
Therapy with FALVIN ® should be preceded by a careful medical examination in order to clarify the infectious origin of the lesions and the relative prescriptive appropriateness.
The patient in therapy should:
- avoid contact of the drug with the eyes;
- thoroughly cleanse the skin region to be treated before applying the drug;
- clean hands thoroughly after application to avoid residues;
- avoid direct exposure to ultraviolet radiation;
- scrupulously follow the medical indications.
in order to optimize the therapeutic efficacy of the drug and limit the incidence of side effects.
PREGNANCY AND BREASTFEEDING
The absence of studies able to fully characterize the safety profile of Fenticonazole for fetal health, extends the aforementioned contraindications to the use of FALVIN ® also to pregnancy and the subsequent breastfeeding period.
Interactions
Drug interactions worthy of clinical note are currently unknown.
Contraindications FALVIN ® Fenticonazole
The use of FALVIN ® is contraindicated in patients who are hypersensitive to the active substance or to one of its excipients.
Undesirable effects - Side effects
The use of FALVIN ®, especially if prolonged for a long time, could determine the appearance of local adverse reactions such as redness, burning and erythema.
Clinically relevant side effects are decidedly more rare.
Note
FALVIN ® is a prescription drug prescribed by a doctor.