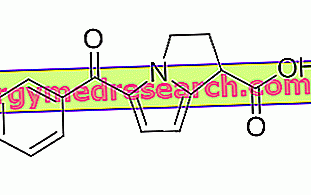
VERMOX ® is a drug based on Mebendazole
THERAPEUTIC GROUP: Antiparasitic
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications VERMOX ® Mebendazole
VERMOX ® is a broad-spectrum antiparasitic drug used in the treatment of infections caused by roundworms, pinworms, whipworms, hookworms, strongyloids and tapeworms.
Mechanism of action VERMOX ® Mebendazole
VERMOX ® is a medicinal specialty, widely used in clinical settings especially in the treatment of pinworm infestations, based on Mebendazole, an active ingredient with a broad spectrum of action directed towards the main intestinal parasites of humans.
Thanks to the low systemic absorption that characterizes it, useful both in limiting the potential systemic effects of the drug and in optimizing intestinal activity, Mebendazole actively carries out its own biological function by binding the tubulin of the parasite and preventing the polymerization of microtubules.
Such ultrastructural modifications can also affect the normal metabolic activity of the parasite, which cannot effectively capture glucose, undergoes a series of energy alterations that seriously compromise its vital capacity to the point of inducing its death through autolytic processes.
What remains of the parasite will be eliminated with the feces along with the drug, while the small amount absorbed by the gastro-enteric mucosa following a first-pass metabolism can be rapidly excreted.
Studies carried out and clinical efficacy
MEBENDAZOLO AND GIARDIASI
World J Gastroenterol. 2006 Oct 21; 12 (39): 6366-70.
Study demonstrating the efficacy of Mebendazole in the treatment of Giardiasis in patients aged between 5 and 15 years, without further improvements deriving from more intense therapies than those classically used.
MEBENDAZOLE AND ALBENDAZOLE IN THE TREATMENT OF TENIASIS
PLoS One. 2011; 6 (9): e25003.
Clinical trial that reaffirms the efficacy of Mebendazole, at high doses, in the treatment of teniasis and intestinal parasites, while describing the greater efficacy of other pesticides of more recent formulation such as Albendazole.
MEBENDAZOLO AND PREGNANCY
Congenit Anom (Kyoto). 2005 Sep; 45 (3): 85-8.
Clinical trial conducted on only a small number of patients, which did not identify a teratogenic and fetotoxic potential of Mebendazole for the embryo or the fetus. Despite the result, the same authors do not exclude these activities given the low number of patients examined.
Method of use and dosage
VERMOX ®
Tablets for oral use 100 mg - 500 mg of Mebendazole;
Oral suspension of 20 mg of Mebendazole per ml of solution.
The dosage, the pharmaceutical form and the duration of the therapy must be defined by the physician according to the patient's physiopathological conditions, the severity of the clinical picture and above all according to the type of parasite responsible for the pathology.
In principle, the therapy is always of short duration, generally of a few days, and is characterized by dosages ranging from 100 to 500 mg daily.
Warnings VERMOX ® Mebendazole
VERMOX ® therapy should be preceded by a careful medical examination in order to assess the prescriptive appropriateness of Mebendazole and the possible presence of contraindications to the use of the drug.
In addition to drug therapy, the patient, under appropriate medical advice, should also apply all the hygienic rules useful for limiting the spread of the parasite in the environment.
Rarely and especially in children under one year of age hypersensitivity reactions, sometimes even serious, have been documented.
It is also important to remember that this drug is not effective against cysticercosis.
PREGNANCY AND BREASTFEEDING
The teratogenic and mutagenic potential of Mebendazole, observed on experimental models, extends the aforementioned contraindications to the use of VERMOX ® also to pregnancy and the subsequent breastfeeding period.
Interactions
The intense first-pass metabolism to which the small amount of Mebendazole absorbed by the intestinal mucosa is subjected, could be compromised by the simultaneous intake of active ingredients with inhibitory action such as cimetidine, thus inducing an increase in the blood concentrations of the drug.
The presence of control case studies, in which the appearance of severe dermatolytic reactions is reported, imposes a ban on the simultaneous intake of Mebendazole and Metronidazole.
Contraindications VERMOX ® Mebendazole
The use of VERMOX ® is contraindicated in case of hypersensitivity to the active ingredient or to one of its excipients.
Undesirable effects - Side effects
Treatment with Mebendazole could lead to the appearance of adverse reactions, fortunately rare, affecting the gastro-enteric system with diarrhea, abdominal pain, nausea and vomiting.
The reactions to the haematopoietic apparatus or those related to hypersensitivity are decidedly more rare.
Note
VERMOX ® is a prescription-only drug.