Fats or Lipids
Lipids are ternary organic substances insoluble in water and soluble in apolar solvents such as ether and benzol.
From the nutritional point of view they are divided into:
- STORAGE LIPIDS (98%), with energy function (triglycerides);
- CELL LIPIDS (2%), with a structural function (phospholipids, glycolipids, cholesterol).
From the chemical point of view they are divided into:
- SAPONIFIABLE OR COMPLEX: can be split, by hydrolysis, into fatty acids and molecules carrying one or more alcohol groups (glycerides, phospholipids, glycolipids, waxes, sterids);
- NON SAPONIFICABLE OR SIMPLE: they do not contain fatty acids in their structure (terpenes, steroids, prostaglandins).
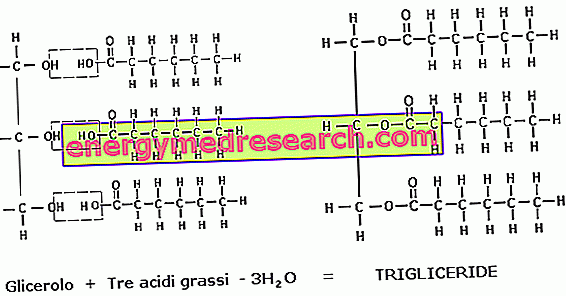
In the human body and in the foods that feed it, the most abundant lipids are triglycerides (or triacylglycerols). They are formed by the union of three fatty acids with a glycerol molecule.
LEGEND:

The carboxyl group is called the functional group of an organic molecule consisting of an oxygen atom bound with a double bond to a carbon atom which is also bound to a hydroxyl group (-OH).
Fatty acids
Fatty acids, fundamental components of lipids, are molecules made up of a chain of carbon atoms, called the aliphatic chain, with a single carboxyl group (-COOH) at one end. The aliphatic chain that constitutes them tends to be linear and only in rare cases is it presented in a branched or cyclical form. The length of this chain is extremely important, as it affects the physical-chemical characteristics of the fatty acid. As it lengthens, the solubility in water decreases and consequently increases the melting point (greater consistency).
Fatty acids generally have an even number of carbon atoms, although in some foods, such as vegetable oils, we find minimal percentages with odd numbers.
In the human body fatty acids are very abundant, but rarely free and mostly esterified with glycerol (triacylglycerols, glycerophospholipids) or with cholesterol (cholesterol esters).
Since each fatty acid is formed by an aliphatic (hydrophobic) carbonaceous chain which

This feature heavily affects the entire digestive process of lipids.
Based on the presence or absence of one or more double bonds in the aliphatic chain, the fatty acids are defined:
- saturated when their chemical structure does not contain double bonds,
- unsaturated when one or more double bonds are present
Cis and trans fatty acids

Based on the position of the hydrogen atoms associated with the carbons involved in the double bond, a fatty acid can exist in nature in two forms, a cis and a trans.
The presence of a double bond in the aliphatic chain implies the existence of two conformations:
- cis if the two hydrogen atoms bound to the carbons engaged in the double bond are placed on the same plane
- trans if the spatial arrangement is opposite.
The cis form lowers the melting point of the fatty acid and increases its fluidity.

Two equal fatty acids, but which have a cis conformation and a trans conformation bond, have different names. The figure shows a fatty acid at eighteen carbon atoms, with unsaturation in position nine and cis conformation (oleic acid, the most abundant fatty acid in nature and present mainly in olive oil); its trans isomer, present in very low percentages, takes on a different name (elaidic acid).
Importance of the stereo-isomerism of the double bond

Let's look at the image; on the left is a saturated fatty acid, note the perfectly linear aliphatic chain (lipophilic tail).
To his right we see the same fatty acid with a trans-type bond. The chain undergoes a small flexion, but still remains a linear structure, similar to that of saturated fatty acid.
Further to the right we can appreciate the folding of the chain induced by the presence of a double cis bond. Finally, on the far right, there is the very strong folding associated with the presence of two double unsaturated cis bonds.
This explains why butter, a food rich in saturated fatty acids, is solid at room temperature, while oils, in which cis unsaturated fatty acids predominate, are liquids under the same conditions. In other words, the presence of double cis bonds lowers the melting point of the lipid.
Where are the trans fatty acids found?
In order to give greater consistency to oils and unsaturated fats, processes (hydrogenation) have been devised in which the artificial breakage of a double bond and the hydrogenation of the product is carried out, thus obtaining foods in which the percentage of the trans form is high.
As already mentioned, natural unsaturated fats are normally found in the cis form. However, a small amount of trans fat is present in the food, as it is formed in the stomachs of ruminants due to the action of certain bacteria. For this reason, in milk, dairy products and beef there are very small quantities of trans fatty acids. The same are also found in the seeds and leaves of various plants, whose food consumption is irrelevant.
The greatest health risks derive from the massive use of hydrogenated oils and fats, which abound above all in margarines, sweet snacks and in many spreadable products. This process takes place through the use of specific catalysts which subject the mixture of animal oils and fats to high temperatures and pressures, up to obtaining chemically altered fatty acids. This process is particularly tempting for the food industries since it allows to obtain fats at a reduced cost and with specific requirements (spreadability, compactness, etc.). Furthermore, the storage time is considerably extended, a fundamental aspect also from an economic point of view.
Why are trans fatty acids dangerous?
All this attention given to trans fatty acids (trans fatty acid) is due to the negative health implications that their use entails. In fact, these fatty acids cause an increase in "bad cholesterol" (LDL lipoprotein) accompanied by a decrease in the "good" fraction (HDL lipoprotein). A high consumption of trans fatty acids, strongly represented in margarine and baked goods (snacks, spreads, etc.), therefore increases the risk of developing serious cardiovascular diseases (atherosclerosis, thrombosis, stroke, etc.).
What are NON-hydrogenated vegetable fats?
Today, the food industry is able to use alternative technologies to hydrogenation, to obtain vegetable fats lacking dangerous trans fatty acids, but with the same organoleptic characteristics.
In any case, these are products artificially handled, not natural and perhaps made from poor quality or already rancid oils. Furthermore, they still have a high content of saturated fatty acids, precisely because they are semi-solid at room temperature.
Nomenclature of fatty acids
The nomenclature of fatty acids is very important, although quite complex and in some respects controversial.
First of all it is necessary to quantify the length of the aliphatic chain, expressing it with the letter C followed by the number of carbons present in the fatty acid (eg C14, C16, C18, C20 etc.).
Secondly it is necessary to indicate the number of unsaturations, following the symbol Cn with the symbol ":" followed by the number of double or triple bonds (for example, oleic acid, having a chain of 18 carbon atoms in which a only unsaturation, it will be indicated by the initials C18: 1).
Finally, it is necessary to specify where the possible unsaturation is located. In this regard there are two different nomenclatures:
- the first refers to the position of the first unsaturated carbon that is encountered starting to number the carbon chain from the initial carboxylic group; this position is indicated by the initials Δn, where n is, precisely, the number of carbon atoms present between the carboxyl end and the first double bond.
- In the second case the numbering of the carbon atoms starts from the terminal methyl group (CH3); this position is indicated by the initials ωn, where n is, precisely, the number of carbon atoms present between the final methyl end and the first double bond
In the case of oleic acid the complete nomenclature is C18: 1 Δ9 or C18: 1 ω9.
The first numbering is preferred by food chemists, while in the medical field the second is preferred.
Examples:
 | Linoleic acid C18: 2 Δ9, 12 or C18: 2 ω6 Α-linolenic acid C18: 3 Δ9, 12, 15 or C18: 3 ω3 |
Saturated fatty acids
Of general formula CH 3 (CH 2 ) n COOH have no double bonds and therefore cannot bind with any other element. The quantity of carbon atoms present in the aliphatic chain confers substance to the substance, raising the melting point and modifying its appearance at room temperature (solid). They are present both in fats of vegetable origin, and in fats of animal origin, but clearly prevail in the latter.
Main Saturated Fatty Acids and their distribution in nature (Da Chimica Degli Alimenti - Cabras, Martelli - Piccin)
| Number of carbon atoms | Composition | Common name | IUPAC name | Short notice | Fusion point (° C) | Sources in kind |
| 4 | CH 3 (CH 2) 2 COOH | Butyric | butanoic | C4: 0 | -5 | |
| 6 | CH3 (CH2) 4COOH | capric | hexanoic | C6: 0 | -2 | Milk fat, coconut oil |
| 8 | CH3 (CH2) 6COOH | caprylic | octanoic | C8: 0 | 17 | Milk fat, coconut oil |
| 10 | CH3 (CH2) 8COOH | Caprico | decanoic | C10: 0 | 32 | Milk fat, coconut oil, elm seeds (50% of fatty acids) |
| 12 | CH 3 (CH 2) 10 COOH | lauric | dodecanoic | C12: 0 | 44 | Lauraceae seeds, coconut oils |
| 14 | CH3 (CH2) 12COOH | Myristic | tetradecanoic | C14: 0 | 58 | Present in all vegetable and animal oils and fats, milk (8-12%), coconut (15-30%), nutmeg 70-80% |
| 16 | CH3 (CH2) 14COOH | palmitic | hexadecanoic | C16: 0 | 62 | Present in all animal and vegetable fatty oils, tallow and lard (25-30%). palm (30-50%), cocoa (25%) |
| 18 | CH3 (CH2) 16COOH | Stearic | octadecanoic | C18: 0 | 72 | Present in all animal and vegetable oils and fats, tallow (20%), lard (10%), cocoa (35%), vegetable oils (1-5%) |
| 20 | CH3 (CH2) 18COOH | arachidic | eicosanoic | C22: 0 | 78 | Present in all animal oils and fats in limited quantities, only in peanut oil 1-2% |
| 22 | CH3 (CH2) 20COOH | behenic | docosanoic | C22: 0 | 80 | Present in all animal oils and fats in limited quantities, only in peanut oil 1-2% |
| 24 | CH3 (CH2) 22COOH | lignoceric | tetracosanoic | C24: 0 | Present in all animal oils and fats in limited quantities, only in peanut oil 1-2% | |
Fatty acids highlighted in bold are the most important from a nutritional point of view. The melting point is directly proportional to the number of carbon atoms present in the fatty acid; for this reason foods rich in long chain fatty acids have a greater consistency.
 | B.C. Lauricus (12: 0) | ||
B.C. Miristico (14: 0) | |||
B.C. Palmiticus (16: 0) | |||
| B.C. Stearic (18: 0) | |||
Saturated fatty acids and health
Saturated dietary fatty acids increase cholesterol levels, so they are atherogenic. It is useful to remember, in this regard, that saturated fatty acids do not all have the same atherogenic power. The most dangerous are the palmitic (C16: 0), the myristic (C14: 0) and the lauric (C12: 0). Stearic acid (C18: 0), on the other hand, despite being saturated, is not very atherogenic, since the body desires it rapidly forming oleic acid.
Even medium chain fatty acids have no atherogenic power.
second part "



