Imipramine (also known as melipramine) is an antidepressant drug of the dibenzoazepine type, belonging to the class of tricyclic antidepressants (TCA).
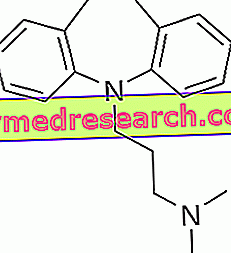
Imipramine - Chemical Structure
Imipramine was discovered in 1950 by the Swiss psychiatrist Ronald Kuhn and became the forefather of TCAs.
Indications
For what it uses
The use of imipramina is indicated for the treatment of:
- Major depressive disorder;
- Depressive phase of manic-depressive psychosis;
- Reactive depression;
- Masked depression;
- Depression in schizophrenic psychosis;
- Involutive depression;
- Severe depression during neurological diseases or other organic diseases;
- Nocturnal enuresis (involuntary emission of urine during the night).
Warnings
Depression is a disease that increases the risk of suicidal thoughts, self-injurious behavior and suicide. After taking imipramine it may take some time before the drug performs its pharmacological action. Therefore, it is necessary to monitor patients carefully until a significant improvement in the depressive state occurs.
Imipramine should not be used in children and adolescents under 18 years of age.
Caution should be exercised in the administration of imipramine in patients suffering from pre-existing cardiovascular diseases, especially in those patients suffering from cardiovascular insufficiency.
The administration of imipramine in subjects with epilepsy - or who have convulsive disorders - should only be performed under strict medical supervision.
Imipramine can cause psychotic states to increase in patients with schizophrenia.
At the beginning of treatment with imipramine in patients suffering from panic attacks, an intensification of anxiety may occur; this paradoxical effect, however, disappears with the continuation of the therapy.
Attention should be paid to the administration of imipramine in patients with a history of glaucoma or increased intraocular pressure.
Particular caution should be used in the administration of imipramine in patients suffering from hepatic, renal and / or tumors of the adrenal glands, as hypertensive crises may occur.
Much attention must be paid to the intake of imipramina by hyperthyroid patients or those taking thyroid hormones, as there may be a worsening of the cardiac side effects induced by imipramine.
During the administration of imipramine it is good to perform periodic checks of the blood count, in particular of the white blood cells.
Abrupt discontinuation of imipramine treatment should be avoided due to adverse effects that may occur.
Interactions
Concurrent administration of imipramine with other antidepressant drugs, such as monoamine oxidase inhibitors ( MAOIs ) should be avoided due to the serious side effects that may occur.
Concomitant administration of imipramine and antidepressants with selective serotonin reuptake inhibitors may cause increased side effects. In particular, concomitant intake of imipramine and fluoxetine or fluvoxamine may cause an increase in plasma concentration of imipramine itself, with consequent increase in adverse effects.
Imipramine can increase the depressive action on the central nervous system of sedative, hypnotic, anxiolytic and anesthetic drugs.
Imipramine can increase the activity of anticoagulant drugs.
Imipramine toxicity affecting the eye, bladder, intestine and central nervous system may be increased by concomitant administration of phenothiazines (a group of antipsychotic and antihistamine drugs), antihistamines and atropine .
Concomitant administration of imipramine and sympathomimetic drugs may cause an increase in cardiovascular side effects induced by imipramine itself.
Concomitant administration of imipramine and L-dopa (a drug used in the treatment of Parkinson's) may increase the risk of arrhythmias and hypotension.
Imipramine should not be given concomitantly with quinidine-type antiarrhythmic drugs, as they may reduce their effectiveness.
Cimetidine (a drug used for the treatment of gastric ulcer) is able to increase the plasma concentration of imipramine, therefore in case of concomitant administration it is necessary to lower the dose of antidepressant administered.
Side effects
Imipramine can cause various side effects, some even serious. However, each individual reacts differently to therapy based on the sensitivity he has towards the drug. Therefore, the type of side effects and the intensity with which they occur are not necessarily the same in all patients.
The following are the main side effects that may occur following treatment with imipramine.
Changes in blood and bone marrow function
Although it is a rare side effect, imipramine can cause bone marrow depression (myelosuppression) and - consequently - reduced blood cell production.
In particular, leukopenia (ie a decrease in white blood cells in the bloodstream with consequent increased susceptibility to contraction of infections) and thrombocytopenia (ie a decrease in the number of blood platelets, with an increased risk of abnormal bleeding and / or bleeding) .
Furthermore, imipramine can cause purple . This term refers to a set of pathologies characterized by the appearance of small spots on skin, organs and mucous membranes. These spots are a consequence of the breakdown of small blood vessels.
Metabolism and nutrition disorders
Imipramine therapy can cause weight gain, but it can also promote the onset of anorexia.
Endocrine system disorders
Treatment with imipramine may cause the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Psychiatric disorders
Imipramine can cause various psychiatric disorders, including:
- Restlessness and agitation;
- Euphoria;
- Delirium;
- Hallucinations;
- Mania;
- Confusion;
- Anxiety;
- Hypomania;
- Sleep disorders;
- Disorientation.
More rarely, imipramine can trigger aggressive behavior, ideation and / or suicidal behavior.
Nervous system disorders
Imipramine treatment can cause tremors, dizziness, headache, drowsiness, sedation and paresthesia. Furthermore, imipramine can cause convulsions, myoclonia (a short and involuntary contraction of a muscle or a group of muscles), extrapyramidal symptoms (ie Parkinson-like symptoms) and speech disorders.
Eye disorders
Imipramine therapy can cause blurred vision, decreased lacrimation, mydriasis (pupil dilation) and - although rarely - can promote the onset of glaucoma.
Cardiac disorders
Imipramine can cause sinus tachycardia, electrocardiogram abnormalities, arrhythmias, cardiac impulse conduction disturbances, palpitations, heart failure, arrhythmia and ventricular tachycardia, ventricular fibrillation and myocardial infarction.
Vascular disorders
Imipramine treatment can cause hot flushes, vasospasm and increased blood pressure. Furthermore, the drug can induce orthostatic hypotension, ie a sudden lowering of blood pressure when passing from a lying position or sitting to an upright position.
Gastrointestinal disorders
After taking imipramine, nausea, vomiting, diarrhea, dry mouth or constipation may occur. More rarely, imipramine can promote the onset of abdominal disorders, paralytic ileus and tongue ulceration.
Hepatobiliary disorders
Imipramine treatment can cause abnormal liver function tests and - in some cases - can induce hepatitis with or without jaundice.
Skin and subcutaneous tissue disorders
Imipramine can cause hyperhidrosis (excessive sweat secretion), itching, photosensitivity reactions, alopecia and skin hyperpigmentation.
Interruption symptoms
Following abrupt cessation of treatment with imipramine, so-called withdrawal symptoms may occur. The main symptoms that can arise are nausea, vomiting, abdominal pain, diarrhea, insomnia, anxiety, nervousness and headache.
Other side effects
Imipramine can also cause other side effects, including:
- Allergic reactions in sensitive subjects;
- Changes in the glycemic rate;
- Weight loss;
- Tinnitus (ie an auditory disorder characterized by noises such as buzzing, hissing, whistling, etc.);
- Stroke (very rarely);
- Urination disorders and urinary retention;
- Mammary hypertrophy;
- Galactorrhea, ie the abnormal secretion of milk in women who are not breastfeeding;
- Changes in libido;
- Fatigue;
- Asthenia;
- Edema;
- Temperature.
Overdose
There is no specific antidote for imipramine overdose, so treatment is only symptomatic.
The symptoms resulting from a drug overdose consist of an exacerbation of the side effects, especially those affecting the cardiovascular system and the nervous system.
If you suspect you have taken an overdose of medication, you must contact a doctor immediately and go to a hospital. It may be useful to induce vomiting and perform gastric lavage.
Action mechanism
Imipramine is a tricyclic antidepressant capable of inhibiting noradrenaline (NA) reuptake and - more mildly - it also inhibits the reuptake of serotonin (5-HT).
In particular, imipramine hinders the binding of NA and 5-HT with transporters assigned to their reuptake within the presynaptic nerve termination (NET for noradrenaline and SERT for serotonin).
The permanence of noradrenaline and serotonin within the synaptic space for a prolonged time causes these to interact more with their own receptors placed on the postsynaptic nerve termination. The greater receptor interaction of NA and 5-HT results in an increase in the noradrenergic and serotonergic signal; this increase favors the improvement of depressive pathology.
Mode of Use - Posology
Imipramine is available for oral administration in the form of tablets that must be swallowed whole, without chewing.
The dosage of imipramina must be established by the doctor based on the type of pathology to be treated and must be adapted to the patient according to his / her condition and his / her clinical picture.
Below are the dosages usually used.
Depressive disorders
For the treatment of depressive disorders in adults, the usual dose is 25 mg of imipramine to be administered 2-3 times a day. The maximum dose of drug that can be administered is 200-300 mg / day.
In elderly patients, on the other hand, the initial dosage is 10 mg of imipramine per day, which can be increased up to 30-50 mg / day.
Nocturnal enuresis
For the treatment of nocturnal enuresis the dosage of imipramina varies from 25 mg to 75 mg of drug per day, depending on the patient's age.
Pregnancy and breastfeeding
The administration of imipramine in pregnant women - both established and suspected - should be avoided.
Because imipramine is excreted in breast milk, breastfeeding mothers should not take the medicine.
Contraindications
The use of imipramina is contraindicated in the following cases:
- Known hypersensitivity to imipramine or other tricyclic antidepressants belonging to the dibenzoazepine group;
- In case of simultaneous therapy with IMAO;
- In patients with glaucoma;
- In patients suffering from pre-existing gastrointestinal or genitourinary disorders;
- In patients with liver disease;
- In patients suffering from pre-existing cardiovascular diseases;
- In pregnancy and during lactation;
- In children and adolescents under the age of 18 years.