Cefixime belongs to the third generation cephalosporin class. It is a bactericidal antibiotic (ie it is able to kill bacterial cells) of the beta-lactam type.
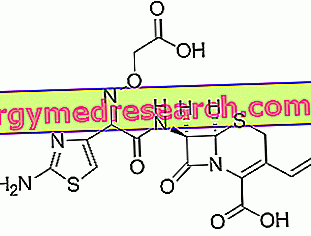
Cefixime - Chemical Structure
Indications
For what it uses
Cefixime is used to treat infections caused by bacteria that are sensitive to it.
More precisely, the use of cefixime is indicated for the treatment of:
- Upper and lower respiratory tract infections, such as pharyngitis, tonsillitis, pneumonia and bronchitis;
- Nasal sinus infections;
- Otorhinolaryngological infections, such as - for example - average otitises;
- Infections of the kidneys and urinary tract;
- Genital infections.
Warnings
Cefixime should be administered with caution in patients who have experienced previous allergic reactions to other cephalosporins, penicillins or other types of antibiotics. In any case, before starting treatment with cefixime, it is good to have identified any patient allergies.
If any hypersensitivity reaction occurs, treatment with cefixime must be stopped immediately.
In patients with renal insufficiency, in patients on hemodialysis or on peritoneal dialysis, the usual dose of cefixime should be reduced.
Caution should be exercised when administering cefixime in patients with a history of gastrointestinal disease, especially in the case of colitis.
The abuse or misuse of cefixime can lead to the development of resistant bacterial strains as well as favor the development of superinfections with resistant bacteria or fungi that are normally present in human bacterial flora (such as, for example, Clostridium difficile infections or from Candida albicans ).
Clostridium difficile is considered the main cause of the occurrence of pseudomembranous colitis. Milder cases of colitis can be resolved simply by stopping treatment, while more severe cases may also require pharmacological treatment.
Cefixime may result in false positives in the test for the determination of glucose in urine (glycosuria) and in the Coombs test.
Interactions
A lot of caution should be used when administering cefixime in patients already treated with coumarin oral anticoagulants (such as warfarin), as cefixime may increase the activity of these drugs. This leads to an increase in prothrombin time and increases the risk of bleeding.
Cefixime may reduce the effectiveness of oral contraceptives, so it is advisable to take additional precautions for the duration of antibiotic therapy.
Nifedipine (an antihypertensive drug) may increase the plasma concentration of cefixime.
Before taking cefixime, you must tell your doctor if you are already taking aminoglycosides, colistin, vancomycin (other antibiotic drugs) or furosemide (a powerful diuretic) due to the possible damage that can occur to the kidneys.
In any case, it is advisable to inform your doctor if you are taking - or have recently been - any type of medication, including over-the-counter medicines and homeopathic and / or herbal products.
Side effects
Cefixime can induce various types of side effects, but not all patients experience them. This depends on the different sensitivity that each individual has towards the drug. Therefore, it is not said that the adverse effects occur all with the same intensity in each patient.
The following are the main side effects that may occur during cefixime therapy.
Allergic reactions
Cefixime - like any other drug - can trigger allergic reactions in sensitive individuals.
These reactions can manifest with symptoms such as:
- Reactions similar to serum sickness;
- arthralgia;
- Medication fever;
- Facial edema;
- Anaphylaxis.
The appearance of any allergic reaction requires immediate discontinuation of cefixime treatment.
Gastrointestinal disorders
Treatment with cefixime may cause:
- Glossitis;
- Nausea and vomit;
- Abdominal pains;
- Heartburn;
- Difficulty of digestion;
- Diarrhea.
The transition from single-dose administration to administration in two divided doses can remedy the problem of diarrhea.
If diarrhea occurs in severe form, on the other hand, it could be a sign of the occurrence of pseudomembranous colitis caused by a superinfection with Clostridium difficile, so it is necessary to immediately inform the doctor.
Blood and lymphatic system disorders
Treatment with cefixime can cause disorders of the hemolymphopoietic system (ie the system responsible for the production of blood cells). Such disturbances can cause:
- Hemolytic anemia;
- Eosinophilia, ie an increase in the blood concentration of eosinophils;
- Plateletopenia (ie the decrease in the number of platelets in the bloodstream), with consequent increased risk of bleeding;
- Leukopenia, ie the reduction in the number of leukocytes in the bloodstream.
Hepatobiliary disorders
Treatment with cefixime may temporarily increase blood levels of transaminases, alkaline phosphatase and bilirubin. Furthermore, the drug can promote the onset of jaundice.
Kidney and urinary tract disorders
Treatment with cefixime can cause a transient increase in azotemia (ie the blood concentration of non-protein nitrogen) and creatinemia (ie blood creatinine concentration).
Lung and respiratory tract disorders
Treatment with cefixime may cause breathing difficulties.
Skin and subcutaneous tissue disorders
Treatment with cefixime may cause:
- Skin rash;
- Urticaria;
- Itch;
- Multi-faceted rashes;
- Drug cutaneous erythema accompanied by eosinophilia and systemic symptoms (commonly known as DRESS, Drug Reaction with Eosynophilia and Systemic Symtomps);
- Stevens-Johnson syndrome;
- Toxic epidermal necrolysis.
Nervous system disorders
Treatment with cefixime can cause headaches and dizziness.
Other side effects
Other side effects that may occur during treatment with cefixime are:
- Temperature;
- Anorexia;
- Candida vaginitis.
Overdose
If you suspect you have taken an overdose of cefixime, you must contact your doctor immediately and contact your nearest hospital.
Action mechanism
Cefixime performs its bactericidal action by interfering with the synthesis of peptidoglycan (the bacterial cell wall).
Peptidoglycan is a polymer made up of parallel chains of nitrogenated carbohydrates, joined together by transverse bonds between amino acid residues. These bonds are formed thanks to the enzyme transammidase.
Cefixime binds to transammidase preventing it from forming the aforementioned bonds. In doing so, weak areas are created within the peptidoglycanic structure that lead to the lysis and death of the bacterial cell.
Mode of Use - Posology
Cefixime is available for oral administration in the form of coated tablets, dispersible tablets and granules for oral suspension.
The drug must be taken at the same time every day and the duration of treatment established by the doctor must be strictly observed.
The following are some indications on the doses of cefixime usually used.
Adults and adolescents over 12 years of age
The dose of cefixime usually administered is 400 mg which can be taken as a single dose or in two divided doses.
Children under 12 years of age
For the treatment of infections in children, usually, granules are used for oral suspension. The dose of cefixime usually administered is 8 mg / kg of body weight to be taken once a day.
Patients suffering from kidney disease
In this category of patients, the doctor may decide to reduce the doses of cefixime usually administered.
Pregnancy and breastfeeding
The use of the drug by pregnant women - established or presumed to be - should be carried out only in cases of real need and only under the strict control of the doctor. However, although there is no evidence on the possible toxicity of cefixime to the fetus, as a precaution it would be better to avoid taking the drug in the first trimester of gestation.
Breast-feeding mothers should seek advice from their doctor to find out if stopping cefixime therapy is necessary to continue breastfeeding, or vice versa.
Contraindications
The use of cefixime is contraindicated in patients with known hypersensitivity to cefixime itself or to other cephalosporins and in patients who have had acute allergic reactions to penicillins or other beta-lactam antibiotics.