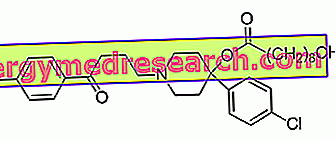
ACTIVELLE ® is a drug based on estradiol hemihydrate + norethisterone acetate
THERAPEUTIC GROUP: Female sexual hormones - Progestin and estrogen, biphasic combination
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications ACTIVELLE ® - Estradiol + Norethisterone
ACTIVELLE ® is commonly used in the pharmacological treatment of menopausal symptoms in post-climacteric women.
The presence of estrogens and progestins also allows to prevent the onset of chronic pathological conditions such as osteoporosis, being therefore used in the prevention of osteoporosis in post-menopausal women at high risk of pathological fractures.
Action mechanism ACTIVELLE ® - Estradiol + Norethisterone
Hormone replacement therapy now uses progestin-related estrus to improve post-climacteric symptoms while safeguarding the patient's state of health.
Menopause is in fact inevitably accompanied by a progressive decline in ovarian function which results in a reduction in the production of estrogen due to various vasomotor disorders, such as hot flashes, and neurological disorders such as insomnia, changes in mood and depression.
The exogenous administration of estrogens allows the control of the aforementioned symptoms, improving the quality of life of post-menopausal women, thus compensating for the physiological fall of these hormones.
However, despite the important and unavoidable therapeutic activity, estrogens in general and estradiol in the first place, present an intense inducing activity towards endometrial cells responsible for hyperplasia and in some cases of carcinomas.
This important risk is fortunately controlled by the simultaneous intake of synthetic progestogens such as norethisterone, which in addition to buffering the proliferative activity of endometrial cells, contribute to the control of climacteric symptoms and to the prevention of osteoporosis.
Studies carried out and clinical efficacy
1. ACTIVELLE AND VASCULAR RISKS
Medium-term study demonstrating that the intake of ACTIVELLE ® does not significantly affect the cardiovascular and cerebrovascular risk, as do other progestagen like ANGELIQ.
2. ACTIVELLE CLINICAL EFFECTIVENESS
Work describing how the administration of ACTIVELLE is effective in reducing menopausal vasomotor symptoms in just 3 months of treatment, ensuring the achievement of normal serum estradiol levels.
3. ACTIVELLE AND MAMMOGRAPHIC DENSITY
The assumption of low dose progestagen estrous, regardless of the dosage scheme used (continuous or cyclical), does not seem to determine any type of mammographic density increase. This parameter is important for reducing the risk of developing neoplastic diseases.
Method of use and dosage
ACTIVELLE ®
1 mg estradiol hemihydrate and 0.5 mg norethisterone acetate coated tablets:
the pharmacokinetic properties of estradiol and norethisterone allow the correct implementation of hormone replacement therapy by taking one tablet a day, every day at the same time.
In this case there should be no suspension periods, as this is a continuous combination therapy.
Warnings ACTIVELLE ® - Estradiol + Norethisterone
The intake of ACTIVELLE ® must necessarily be preceded by a careful medical examination useful for verifying the prescriptive appropriateness and the possible presence of leiomyomas (uterine fibroids) or endometriosis, a history of thromboembolic disorders, risk factors for estrogen-dependent tumors, e.g. hereditary predisposition (1st degree relatives with breast cancer), hypertension, liver disease, diabetes mellitus with or without vascular involvement, cholelithiasis, migraine or headache (severe), systemic lupus erythematosus, history of endometrial hyperplasia, epilepsy, and osteosclerosis, potential risk factors capable of increasing the risk of developing serious side effects associated with estrogen progestagen therapy such as embolic and neoplastic thrombus events.
In these cases it is therefore important that the doctor carefully evaluate the cost / benefit ratio, monitoring the patient's health through periodic checks.
ACTIVELLE ® contains lactose so that its intake, in patients with lactase enzyme deficiency, glucose / galactose or lactose intolerance, may be associated with severe gastrointestinal disorders.
PREGNANCY AND BREASTFEEDING
The absence of clinical trials able to characterize the safety profile of ACTIVELLE ® when taken during pregnancy, and the presence of experimental studies able to demonstrate the toxicity of high estrogenic doses on the health of the fetus extend the contraindications also to pregnancy.
Furthermore, given the ability of estradiol and norethisterone to permeate the breast filter and concentrate in breast milk, it widens the aforementioned contraindication also to the subsequent breastfeeding period.
Interactions
The simultaneous intake of active ingredients inducing cytochromial enzymes such as primidone, phenytoin, barbiturates, carbamazepine (used to treat epilepsy), rifampicin (used to treat tuberculosis), ampicillin, tetracycline, griseofulvin (antibiotics used for the treatment of infectious diseases), ritonavir, modafinil and sometimes St. John's wort (hypericum perforatum), could increase the clearance of estradiol and norethisterone contained in ACTIVELLE ® reducing its therapeutic effects.
It is also useful to remember that the use of sex hormones could cause variations in some laboratory values related to thyroid, hepatic, renal and adrenal function.
Contraindications ACTIVELLE ® - Estradiol + Norethisterone
ACTIVELLE ® is contraindicated in case of hypersensitivity to the active ingredient or to one of its excipients, in case of vaginal bleeding of unknown origin, breast cancer or dependent estrogen-progestin tumors, changes in liver and kidney function, existing or previous thromboembolic processes
Undesirable effects - Side effects
The intake of ACTIVELLE ® as well as that of other progestogen estrus used in hormone replacement therapy, is associated with numerous side effects of varying clinical extent.
Nervousness and alterations in mood, migraine, palpitations, abdominal pain, nausea, occasional bleeding spotting, edema and weight gain are the most common, but clinically controllable, and easily regressive adverse reactions when therapy is stopped.
On the contrary, the increased incidence of episodes of gallbladder lithiasis and pancreatitis, embolic thrombus events and neoplastic pathologies, is currently the main problem related to the ingestion of estrogen progestins for prolonged periods of time and in particular categories of patients at risk.
Note
ACTIVELLE ® is salable only under medical prescription.