Octreotide is a peptide whose structure is similar to that of somatostatin, a hormone that is produced by the hypothalamus, pancreas and gastrointestinal tract. It was synthesized in 1979 by the chemist Wilfried Bauer and was then marketed under the name Sandostatin ®.
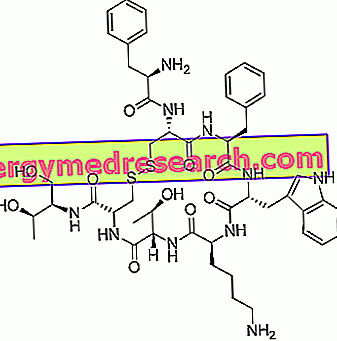
Octreotide - Chemical Structure
Octreotide has no actual anti-tumor activity, but is used to alleviate symptoms caused by certain types of tumors.
Indications
For what it uses
The use of octreotide is indicated for the treatment of:
- Acromegaly in patients in whom surgical treatment is inappropriate or not possible; acromegaly is a disease characterized by excessive production of growth hormone in adulthood;
- Pituitary adenomas secreting growth hormone (also known as GH);
- Pituitary adenomas secreting thyroid-stimulating hormone (also known as TSH);
- To relieve diarrhea and hot flushes associated with the carcinoid syndrome ;
- To relieve symptoms associated with gastroenteropancreatic endocrine tumors, such as - for example - VIPomas, or neuroendocrine tumors characterized by excessive production of vasoactive intestinal hormone, also known as VIP.
Furthermore, octreotide can be labeled with radionuclides such as indium-111 or gallium-68 and used in diagnostic imaging imaging.
If, on the other hand, the drug is labeled with radionuclides such as yttrium-90 or luthium-177, it can be used in the treatment of inoperable neuroendocrine tumors.
Warnings
Since octreotide is able to inhibit insulin synthesis, impairment of glucose tolerance may occur. Blood glucose levels should therefore be carefully monitored.
Octreotide is able to reduce the motility of the gallbladder, so radiographic analyzes are recommended both before and during treatment with the drug.
In some individuals, treatment with octreotide can cause impaired absorption of dietary fat.
Since octreotide can decrease the absorption of vitamin B12, in patients being treated with the drug - suffering from a previous lack of this vitamin - constant monitoring of the blood levels of the same is necessary.
Interactions
Octreotide is able to reduce the intestinal absorption of ciclosporin (an immunosuppressive drug used in the prevention of rejection in transplants) and can delay the absorption of cimetidine (a drug used for gastric ulcer).
Concomitant administration of octreotide and bromocriptine may increase the bioavailability of bromocriptine itself.
Octreotide - like other somatostatin analogues - could reduce drug metabolism such as:
- Quinine, a natural alkaloid with antipyretic, antimalarial and analgesic properties;
- Carbamazepine, a drug used to treat epilepsy;
- Digoxin, a drug used to increase the strength of cardiac contraction;
- Warfarin, an anticoagulant;
- Terfenadine, an antihistamine drug.
We must therefore pay close attention to the simultaneous administration of these drugs and octreotide.
Side effects
Octreotide can induce various side effects that vary depending on the amount of drug administered and depending on the patient's general condition.
It is not certain that all the adverse effects should manifest themselves with the same intensity in each patient, since there is a high variability of response to the therapy between one individual and another.
The following are the main side effects that may occur following octreotide therapy.
Gastrointestinal disorders
Octreotide therapy can cause a variety of gastrointestinal disorders that occur in the form of diarrhea, abdominal pain, nausea, vomiting, constipation, flatulence, abdominal swelling and discoloration of the stool. These effects can be reduced by avoiding food intake near the time of drug administration.
More rarely, acute intestinal obstruction, acute pancreatitis, abdominal muscle defense contracture and cholelithiasis-induced pancreatitis (presence of stones in the bile ducts or gall bladder) may also occur.
Hepatobiliary disorders
Treatment with octreotide may cause cholelithiasis, cholecystitis (an inflammation of the gallbladder, otherwise known as a gall bladder) or hyperbilirubinemia (increased blood concentration of bilirubin).
Octreotide can also cause acute hepatitis without cholestasis, cholestatic hepatitis, jaundice and cholestatic jaundice.
Skin and subcutaneous tissue disorders
Octreotide can cause skin eruptions accompanied by itching, hives and alopecia . The use of neutral detergents is recommended.
Cardiac disorders
Octreotide therapy may favor the appearance of cardiac arrhythmias, both bradycardia and tachycardia type.
Thyroid disorders
Treatment with octreotide can cause hypothyroidism and thyroid dysfunction that occur with a decrease in blood levels of TSH and the hormone L-thyroxine (or T4).
Nervous system disorders
Following the use of octreotide, the appearance of headache and dizziness is very common.
Nutrition and metabolism disorders
Octreotide can alter glucose tolerance and induce hyperglycemia which can become permanent following chronic administration of the drug. Therapy with the drug can also promote the onset of anorexia .
Changes in diagnostic tests
Octreotide therapy may cause an increase in blood levels of transaminases, alkaline phosphatase and γ-glutamyl transferase.
Other side effects
Other side effects that may occur during treatment with octreotide are:
- Allergic and / or hypersensitivity reactions in sensitive subjects;
- Anaphylaxis;
- Dehydration;
- Dyspnoea;
- Localized pain at the injection site of the drug.
Overdose
There is no antidote for overdose. Symptoms that may appear - following an overdose of octreotide - are depression, fatigue, weakness, anxiety, lack of concentration and frequent urination. Drug treatment in case of overdose is only symptomatic.
Action mechanism
As mentioned above, octreotide is a synthetic drug with a structure similar to that of the endogenous somatostatin hormone.
Octreotide has biological effects similar to those of somatostatin, but has a longer duration of action. In particular - compared to somatostatin - octreotide more strongly inhibits the release of growth hormone, glucagon and insulin.
Mode of Use - Posology
For octreotide administration the subcutaneous route is recommended. However, if rapid action is needed - for example, in the case of carcinoid crisis - the drug can be administered diluted in bolus intravenously, in conjunction with constant monitoring of the heart rhythm.
The dose of octreotide to be administered and the duration of treatment must be established by the doctor on the basis of the pathology to be treated and according to the patient's condition and clinical picture.
In patients with pre-existing liver cirrhosis, there may be an increase in the bioavailability of octreotide which could be harmful; consequently, an adjustment of the dose of drug administered may be necessary.
In elderly patients, a reduction in tolerability to octreotide was not noted, therefore, dosage adjustment should not be necessary.
Pregnancy and breastfeeding
Studies have been conducted on animals that have shown a transient delay in offspring growth prior to weaning, but no fetotoxic, teratogenic or other effects on reproduction have been shown. Other animal studies have shown, instead, that octreotide is excreted in breast milk.
In light of these studies, it follows that the intake of octreotide during pregnancy should be avoided, except in the case where the doctor does not consider it strictly necessary.
In order to avoid toxic effects on the suckling child, women taking the drug should not breast-feed.
Contraindications
The use of octreotide is contraindicated in the following cases:
- Known hypersensitivity to octreotide;
- Pregnant;
- During breastfeeding.