See also: coumarin in cosmetics
Coumarins are a family of natural substances widely distributed in the plant world. From the chemical point of view, these are derivatives of 5, 6-benzo-2-pyrone, better known as coumarin.

The term coumarin derives from Coumarona odorata, a South American legume from which the molecule was first isolated in 1820.
In the plant world the coumarins can be found both in free form and in glycosidic form, that is they are bound as aglycone to a sugar part. The great structural heterogeneity of these substances reflects an equally wide pharmacological-therapeutic variability.
Cumarine with phlebotonic action

Coumarins with anticoagulant action
When at the end of the nineteenth century the farmers of North America introduced the odorous clover (meliloto) in their pastures and in the feeding of the cattle, a haemorrhagic epidemic appeared very soon, that only a few years later it turned out to be linked to the use of this new fodder.

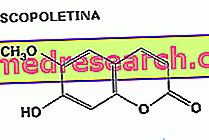

An example of vegetable substances with hypotensive and spasmolytic properties - capable therefore of inhibiting the spastic contraction of the gastro-enteric and genito-urinary smooth muscles associated with cramp-like or colic-type pain - is given by the coumarins of Viburnum prunifolium (scopoletine) and of the Angelica ('essential oil extracted from the roots).

Coumarine with antibacterial and antiviral action
The umbelliferone present in the aerial parts of the Pilosella and in the resins of many Umbelliferae - in addition to being used as a sun screen - has shown interesting antibiotic properties, especially with regard to Brucella, the etiological agent of Brucellosis. The aforementioned esculetin, on the other hand, exhibits bacteriostatic and antifungal properties, while daphnoretin and 3-phenylcumarins have shown anti-hepatitis B and anti-HIV properties respectively.

Coumarine with anti-inflammatory action
Melilotum coumarin promotes tissue healing and regeneration, thanks to its anti-edema, capillarotropic and erythrocyte membrane stabilizing properties (it counteracts the increase in vascular permeability, a very important element in inflammatory phenomena). The esculetin, on the other hand, inhibits the synthesis of prostanoids (prostaglandins, thromboxanes and leukotrienes), molecules involved in asthmatic, allergic and inflammatory reactions.
Cumarine with photosensitizing action

Side effects of coumarins
Particular caution should be taken in the use of dried coumarin-based herbs, due to the already mentioned ability to produce dicumarol in particular situations (see fermentation of the Melilotus). For obvious reasons, these preparations are absolutely contraindicated in patients on anticoagulant (coumadin, sintrom) or anti-platelet therapy (aspirin, clopidogrel etc.). It must be said, however, that coumarin and the other coumarins in themselves do not possess any notable anticoagulant activity, so on a phytotherapeutic level they should not be confused with dicumarol and its therapeutic applications. Devil's claw, boldo, fenugreek and Chinese angelica are examples of coumarin drugs for which important episodes of drug interaction have been reported, with an increase in the anticoagulant activity of drugs such as warfarin.

Among the coumarin derivatives, the aflatoxins produced by the molds of the genus Aspergillus starting from the coumarins, play a very important toxicological role, because they significantly increase the risk of primitive liver cancer. Coumarin itself is moderately toxic to the liver and kidneys.
Finally, due to their potential toxicity, coumarins are contraindicated during pregnancy and lactation.



