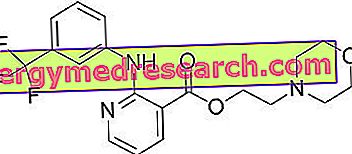
MUCOSOLVAN ® is a drug based on Ambroxol hydrochloride
THERAPEUTIC GROUP: Mucolytics
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Indications MUCOSOLVAN ® Ambroxol
MUCOSOLVAN ® is indicated in the treatment of both acute and chronic respiratory diseases characterized by thick and viscous mucus.
Mechanism of action MUCOSOLVAN ® Ambroxol
Ambroxol, active ingredient of MUCOSOLVAN ®, is a molecule derived from its predecessor Bromexina and equipped with numerous biological activities that make it particularly suitable in the treatment of respiratory diseases.
Firstly the pharmacokinetic properties of Ambroxol allow a rapid gastrointestinal absorption and a prevalent distribution at the bronchopulmonary level, thus allowing the active principle to carry out its therapeutic action at this level improving both the mucociliary clearance and the chemical composition of the mucus itself. making it more fluid, therefore more easily expectorable.
The aforementioned therapeutic action is supplemented by the antioxidant and anti-inflammatory action which allows the active ingredient to protect the respiratory mucosa from the injurious stimuli present in these conditions.
At the end of its activity, generally after a half-life of about 10 hours, Ambroxol, following a hepatic metabolism that determines its glucuronation, is eliminated mainly via the kidney.
Studies carried out and clinical efficacy
AMBROXOLO IN CHROMOSAL PULMONARY OBSTRUCTIONS
Pulm Pharmacol Ther. 2004; 17 (1): 27-34.
Clinical trial demonstrating how prolonged use of Ambroxol can be effective in reducing the re-exacerbation of severe respiratory symptoms in patients suffering from chronic obstructive pulmonary diseases, thus improving the quality of life of these patients.
AMBROXOLO INDUCES SURFACTANT PRODUCTION
Toxicol Appl Pharmacol. 2005 Feb 15; 203 (1): 27-35.
Molecular study that evaluates the ability of Ambroxol to remodulate the biosynthetic capabilities of second-type pneumocytes, inducing the expression of genes involved in Surfactant synthesis. This mechanism of action represents one of the key activities in the treatment with Ambroxol.
HEMOLYTIC ANEMIA INDUCED BY AMBROXOLO
Rev Med Interne. 2006 Oct; 27 (10): 797-8. Epub 2006 Jun 2.
Case report denouncing the onset of hemolytic anemia in a 68-year-old patient treated with Ambroxol, in which an IgA mediated reaction developed.
Method of use and dosage
MUCOSOLVAN ®
Ambroxol hydrochloride 30 mg tablets;
Extended release capsules of 75 mg Ambroxol hydrochloride;
Suppositories of 30 - 60 mg of Ambroxol hydrochloride;
Ambroxol hydrochloride syrup at 0.3%.
The choice of the format and dosage of Ambroxol to be used is the responsibility of the doctor following the evaluation of the patient's general health conditions and the severity of his / her clinical picture.
In general, in adults the use of 90 mg of Ambroxol hydrochloride per day, divided into 3 different administrations, is effective in a few days of treatment to guarantee a prompt remission of symptoms.
Warnings MUCOSOLVAN ® Ambroxol
The use of MUCOSOLVAN ® should be preceded by a careful medical examination in order to assess both the prescriptive appropriateness and the possible presence of potentially incompatible or dangerous conditions for the simultaneous administration of Ambroxol.
More precisely, hepatic and renal diseases, peptic ulcers, respiratory difficulties and difficulty in expectoration are some of the noteworthy clinical conditions for which the use of Ambroxol could be potentially problematic.
In very rare cases, clinically relevant adverse reactions to hypersensitivity to the active ingredient have been observed, requiring treatment to be discontinued.
MUCOSOLVAN ® in tablets contains lactose, therefore its use is not indicated in patients with galactose intolerance, lactase enzyme deficiency and glucose - galactose malabsorption syndrome.
PREGNANCY AND BREASTFEEDING
The aforementioned contraindications to the use of MUCOSOLVAN ® also extend to pregnancy and the subsequent period of breastfeeding, given the ability of Ambroxol to cross the placental barrier and the breast filter, exposing themselves in significant concentrations to the fetus and to the infant.
Interactions
Although no clinically relevant drug interactions are known, it should be remembered that Ambroxol could increase the concentrations of certain antibiotics in bronchopulmonary secretions and saliva, without compromising their clinical efficacy.
Contraindications MUCOSOLVAN ® Ambroxol
The use of MUCOSOLVAN ® is contraindicated in patients who are hypersensitive to the active substance or to one of its excipients, in patients with gastro-duodenal ulcer, in patients suffering from severe liver and kidney diseases and in small patients younger than 2 years.
Undesirable effects - Side effects
The use of MUCOSOLVAN ® may cause adverse reactions such as diarrhea, nausea, vomiting, oral hypoaesthesia and dry mouth.
Fortunately, rarer side effects are noteworthy as those of a neurological nature or hypersensitivity to the active ingredient.
Note
MUCOSOLVAN ® is a non-prescription drug.