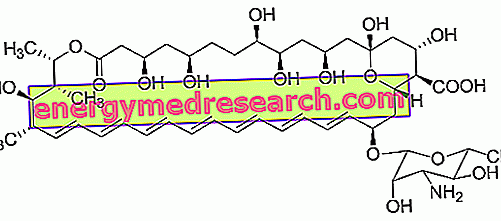
EUMOVATE ® is a drug based on Clobetasone Butyrate
THERAPEUTIC GROUP: Non-associated corticosteroids, dermatological preparations
IndicationsAction mechanismStudies and clinical effectiveness Usage and dosage instructionsWarnings Pregnancy and lactationInteractionsContraindicationsUndesirable effects
Directions EUMOVATE ® Clobetasone
EUMOVATE ® is indicated in the treatment of cutaneous inflammatory pathologies sensitive to corticosteroid therapy such as atopic and seborrheic eczema, diaper dermatitis, localized dermatoses, first-degree burns and sunburn.
Mechanism of action EUMOVATE ® Clobetasone
EUMOVATE ® is a medicinal product based on Clobetasone, a moderately active corticosteroid, particularly effective in the topical treatment of inflammatory skin diseases, given the advantageous pharmacokinetic properties and in particular the very low systemic absorption rate of the drug that follows its topical application. .
Like other corticosteroids, Clobetasone also carries out its therapeutic activity:
- inhibiting the recruitment of inflammatory and allergic cells such as mast cells;
- inhibiting the activation of cellular events involved in the synthesis of inflammatory mediators such as prostaglandins;
- reducing overall the inflammatory stimulus present in the site.
All the aforementioned activities result in an anti-inflammatory, anti-edema, anti-itching and vasoconstrictor action.
Studies carried out and clinical efficacy
contact dermatitis TO CLOBETASONE
Contact Dermatitis. 2000 May; 42 (5): 305.
Clinical case that reports the appearance of severe allergic contact dermatitis following topical application of Clobetasone, thus highlighting the need for medical supervision during treatment.
CLOBETASONE IN CLINICAL PRACTICE
J Dermatolog Treat. 2003 Jun; 14 (2): 71-85.
Very interesting review that summarizes all the potential therapeutic indications of Clobetasone, emphasizing its effectiveness even in the treatment of syndromes characterized by a typical inflammatory course and safety of use.
CLOBETASONE IN THE EYE CLINIC
Eur J Ophthalmol. 2013 May-Jun; 23 (3): 368-76. doi: 10.5301 / ejo.5000229. Epub 2012 Nov 19.
Work that broadens the classic therapeutic indications of Clobetasone also to the eye sector, successfully testing the treatment of inflammatory eye diseases with eye drops at a very low Clobetasone concentration.
Method of use and dosage
EUMOVATE ®
Cream with 0.05% Clobetasone butyrate.
Generally, always according to medical indications, the application of the appropriate quantity of cream is recommended, directly on the region affected by the inflammatory process from 2 to 4 times a day, taking care to gently massage the region in order to ensure complete absorption.
Sometimes the doctor could evaluate the possibility of resorting to occlusive bandaging techniques, so as to increase the degree of absorption of the drug.
Warnings EUMOVATE ® Clobetasone
Topical therapy with corticosteroids must necessarily be preceded by a careful medical examination in order to clarify the origin of the lesions and the prescriptive appropriateness.
The patient in therapy therefore should respect a series of norms, mainly sanitary-sanitary, useful to limit the incidence of potential side effects and to optimize at the same time the effectiveness of the therapy itself.
For this reason the physician should provide all the indications of the case taking care to underline the potential side effects, so that the patient can provide for rapid recognition in order to avoid more serious clinics.
It is also worth remembering that particular categories of patients, such as the elderly and children, may be further more sensitive to the potential side effects of topical corticosteroid therapy, developing far more serious pathological consequences.
PREGNANCY AND BREASTFEEDING
In light of some experimental studies that demonstrate the teratogenic and toxic potential of Clobetasone taken during pregnancy for the health of the fetus, it is necessary to extend the aforementioned contraindications to the use of EUMOVATE ® also to pregnancy and the subsequent breastfeeding period.
Interactions
At the present time, known pharmacological interactions are not known, although it should be remembered that the simultaneous intake of cytochrome inhibitor drugs may increase blood concentrations of the amount of corticosteroid absorbed.
Contraindications EUMOVATE ® Clobetasone
The use of EUMOVATE ® is contraindicated in patients hypersensitive to the active ingredient or to one of its excipients, in patients suffering from inadequately treated viral, bacterial and fungal infections, from rosacea, acne vulgaris and itching without inflammation.
Undesirable effects - Side effects
Treatment with EUMOVATE ®, especially if prolonged over time or if carried out on particular categories of patients at risk, could lead to the appearance of unpleasant local side effects such as redness, burning, hives, hypertrichosis, skin atrophy and erythema.
All potential systemic side effects are far rarer.
Note
EUMOVATE ® is a prescription-only drug.